
 Copy to clipboard
Copy to clipboard 
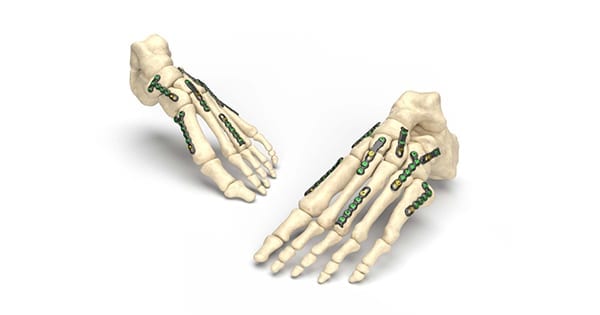
Nextremity Solutions received FDA 510(k) clearance to market the Stratum® RS (Reduced Size) Foot Plating System.
Stratum RS is intended for stabilization and fixation of small bone fragments in fresh fractures, revision procedures, joint fusion and reconstruction of small bones of the foot and ankle, particularly in osteopenic bone.
Stratum RS extends the Stratum Foot Plating system that launched in 2019. The system includes 18 plates that are designed to fit various anatomies in smaller patients. This includes Single Lisfranc, Peanut, Straight, Hockey Stick, L-shaped, Y-shaped and T-shaped plates.
This expansion will result in close to 70 options to treat deformities and fractures in the foot, and includes a single-use disposable instrument kit which eliminates the need for sterilization by hospitals.
Ryan Schlotterback, Chief Technology Officer for Nextremity Solutions said, “We’ve been very pleased with the performance and adoption of our Stratum Foot Plating System. Our new Stratum RS is a wonderful complement to our existing system and will be very familiar to our surgeon customers in both product technology and overall surgical efficiency. The team continues to work hard with our surgeon partners to accelerate the expansion of the Stratum portfolio and we look forward to addressing other anatomical applications.”
Nextremity Solutions received FDA 510(k) clearance to market the Stratum® RS (Reduced Size) Foot Plating System.
Stratum RS is intended for stabilization and fixation of small bone fragments in fresh fractures, revision procedures, joint fusion and reconstruction of small bones of the foot and ankle, particularly in osteopenic bone.
Stratum...
Nextremity Solutions received FDA 510(k) clearance to market the Stratum® RS (Reduced Size) Foot Plating System.
Stratum RS is intended for stabilization and fixation of small bone fragments in fresh fractures, revision procedures, joint fusion and reconstruction of small bones of the foot and ankle, particularly in osteopenic bone.
Stratum RS extends the Stratum Foot Plating system that launched in 2019. The system includes 18 plates that are designed to fit various anatomies in smaller patients. This includes Single Lisfranc, Peanut, Straight, Hockey Stick, L-shaped, Y-shaped and T-shaped plates.
This expansion will result in close to 70 options to treat deformities and fractures in the foot, and includes a single-use disposable instrument kit which eliminates the need for sterilization by hospitals.
Ryan Schlotterback, Chief Technology Officer for Nextremity Solutions said, “We’ve been very pleased with the performance and adoption of our Stratum Foot Plating System. Our new Stratum RS is a wonderful complement to our existing system and will be very familiar to our surgeon customers in both product technology and overall surgical efficiency. The team continues to work hard with our surgeon partners to accelerate the expansion of the Stratum portfolio and we look forward to addressing other anatomical applications.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







