
 Copy to clipboard
Copy to clipboard 
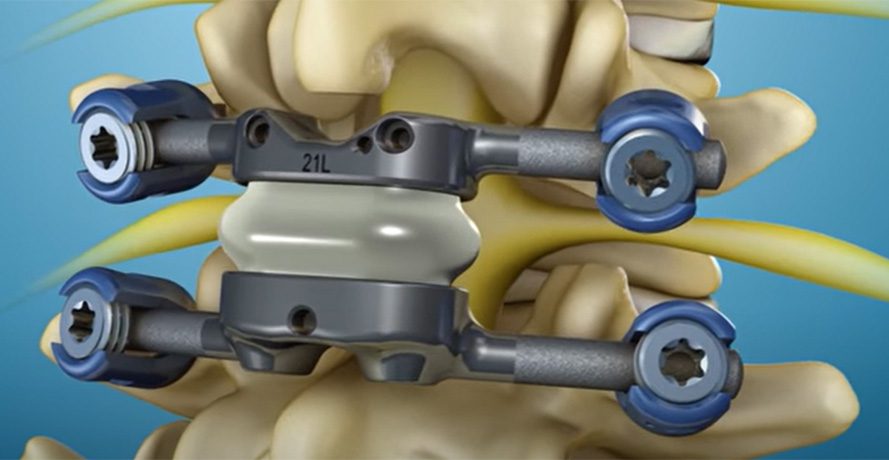
Premia Spine announced the initiation of the Centers for Medicare & Medicaid Services (CMS) New Technology Add-on Payment (NTAP) Program for the TOPS System. The payment, of up to an additional $11,375 beyond the standard DRG payment for the procedure, is available to all facilities that provide inpatient care to patients with spinal stenosis and spondylolisthesis.
This is only the third time that CMS has granted an incentive payment to facilities for the use of any spinal implant.
FDA designated TOPS as a Breakthrough Technology and granted Premarket Approval to the TOPS System with a “superior-to-fusion” claim, underscoring its safety and efficacy. The TOPS System provides patients with spinal stenosis and spondylolisthesis unparalleled advantages over lumbar fusion, including enhanced mobility, decreased post-operative neuro deficit, and more rapid and sustained return to normal activity.
Ron Sacher, CEO of Premia Spine commented, “We are honored by the recognition of CMS. TOPS has proven its ability to provide both clinical excellence and healthcare economic advantage to patients, providers, and payers. The NTAP program is an example of forward-thinking policy that aligns incentives so that patients receive the best short-term and long-term care.”
Source: Premia Spine
Premia Spine announced the initiation of the Centers for Medicare & Medicaid Services (CMS) New Technology Add-on Payment (NTAP) Program for the TOPS System. The payment, of up to an additional $11,375 beyond the standard DRG payment for the procedure, is available to all facilities that provide inpatient care to patients with spinal stenosis...
Premia Spine announced the initiation of the Centers for Medicare & Medicaid Services (CMS) New Technology Add-on Payment (NTAP) Program for the TOPS System. The payment, of up to an additional $11,375 beyond the standard DRG payment for the procedure, is available to all facilities that provide inpatient care to patients with spinal stenosis and spondylolisthesis.
This is only the third time that CMS has granted an incentive payment to facilities for the use of any spinal implant.
FDA designated TOPS as a Breakthrough Technology and granted Premarket Approval to the TOPS System with a “superior-to-fusion” claim, underscoring its safety and efficacy. The TOPS System provides patients with spinal stenosis and spondylolisthesis unparalleled advantages over lumbar fusion, including enhanced mobility, decreased post-operative neuro deficit, and more rapid and sustained return to normal activity.
Ron Sacher, CEO of Premia Spine commented, “We are honored by the recognition of CMS. TOPS has proven its ability to provide both clinical excellence and healthcare economic advantage to patients, providers, and payers. The NTAP program is an example of forward-thinking policy that aligns incentives so that patients receive the best short-term and long-term care.”
Source: Premia Spine

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







