
 Copy to clipboard
Copy to clipboard 
A host of new fracture repair technologies seek to serve the diverse unmet needs of patients and surgeons. Innovations in this space treat the entire fracture site, preventing infection and minimizing the need for surgery. We estimate that the global trauma market reached $8.3 billion in 2023. In this article, we highlight three companies that seek to capture a portion of the market with novel approaches to fracture repair.
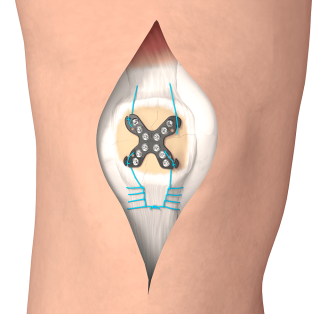
Endeavor Orthopaedics combines the plate, screws and suture into one kit, which allows surgeons to accommodate fracture fixation and soft tissue tasks with a single implant.
Endeavor Orthopaedics Focuses on Bone and Soft Tissue
Endeavor Orthopaedics has developed a patellar fixation solution that is the only product of its kind “with built-in soft tissue management provided in a single-use kit,” according to the company. By combining the plate, screws and suture into one kit, surgeons are able to accommodate fracture fixation and soft tissue tasks with a single implant.
The Summit Patella Plating System offers a biomechanical advantage during patient recovery by off-loading strain onto a single, interconnected construct. The device is FDA 510(k)-cleared and has been shown in clinical studies to lower reoperation rates and instances of the loosening and migration of hardware.
“Other device companies have patella plates on the market, but no one else has one that comes with a sterile and integrated suture,” said Endeavor Orthopaedics’ CEO Greg Parranto. “It’s a technology for any type of avulsion fracture where soft tissue management is needed to solve the repair.”
Inventor, surgeon and company Founder Brent Norris, M.D., was inspired to develop the device in 2011 after being unsatisfied with the uses and outcomes of other options on the market.
“By only treating the bony element of the injury with cannulated screws and orthopedic wire, we were not fully addressing the soft tissue component of the injury or, more importantly, the forces of the extensor mechanism,” Dr. Norris said. “Until these components are addressed, surgeons aren’t optimizing repairs.”
Patella fracture repair is a small market, but one that needs better solutions and can demonstrate the importance of treating bone and soft tissue issues at the fixation site, Mr. Parranto said. The company is raising Series B funding, which will be allocated to further develop its product pipeline and commercial model.

ABL Medical, a subsidiary of IlluminOss Medical, has shown that it’s proprietary multi-frequency illumination system can eliminate different species of bacteria.
IlluminOss Medical Advances Antimicrobial Device
Launched in 2018, IlluminOss’ Bone Stabilization System was recently shown to be adapted for antimicrobial applications. The platform is designed to prevent and treat pathological fractures of the humerus, radius and ulna caused by metastatic bone disease and fragility. It uses light-curable liquid monomer stored in an expandable catheter to stabilize the bone.
Data shared by ABL Medical, a subsidiary of IlluminOss Medical, confirms that its fiber-optic blue light tech can be tailored for antimicrobial use in common medical devices. The device’s proprietary multi-frequency illumination system can eliminate different species of bacteria by disrupting their oxygen-transportation molecules, which initiates a chain reaction that results in the breakdown of cells. Blue light impacts microbes so quickly and entirely that they don’t have time to develop resistance, like they can with conventional antibiotic treatments, according to ABL Medical.
The system, which ABL began developing in 2015, includes an optical light fiber that can emit blue light within a catheter or cannulated medical instrument to kill bacteria. This feature could potentially allow for the application of blue light for new and different orthopedic uses that may not have been previously possible. The system is installed through a small, minimally invasive percutaneous incision. After the implant is cured and polymerized, it uniquely conforms to a patient’s intramedullary canal.
Numerous patents have been issued for the ABL system. The company is collecting clinical data to present to FDA to determine the best regulatory pathway, said Robert Rabiner, Chief Technology Officer and Founder of IlluminOss.

PolyArmor is the first non-invasive external fixation system that’s specifically indicated for wrist fractures
Cambridge Orthopaedic Labs Takes Non-Invasive Approach
Conventional external fixators are fitted into bone with plates, rods and screws. Cambridge Orthopaedic Labs’ PolyArmor instead stabilizes fractures with a set of linkages that can be adjusted to fit a patient’s unique anatomy.
“PolyArmor is the first non-invasive external fixation system that’s specifically indicated for wrist fractures,” said David Anderson, CEO of Cambridge Orthopaedic Labs. “However, the technology has global application in fracture repair and can be used in other areas of the body.”
PolyArmor consists of three cuffs, which slip over the hand, the fractured area of the wrist and the upper forearm. The cuffs are connected by adjustable carbon fiber rods. The device is radiolucent, which allows physicians to set fractures under fluoroscopy or x-ray. The linkages and underlying padding hold reduced fractures in place for the duration of the healing.
Mr. Anderson said that the system is “infinitely adjustable” to the anatomy of the wrist and that the company’s single SKU can fit roughly 90% of the global population. “By adjusting the linkages, surgeons can apply the equivalent of three-point fixation to a fracture without performing surgery,” he added.
PolyArmor received FDA 510(k) clearance in 2022 and was launched in March 2023. The technology was developed by company Founder Ali Bajwa, M.D., F.R.C.S., who experimented with many different configurations before ultimately choosing a linkage system to manage fractures.
Dr. Bajwa wants to deliver surgery-free wrist fracture management to vulnerable populations “because most people who break their wrists are under the age of 14 and over the age of 60,” Mr. Anderson noted. The linkages and padding are also completely immersible and can be worn while patients shower and swim.
By healing simple and complex wrist fractures without surgery, PolyArmor also lessens physicians’ workloads and limits healthcare spending.
A host of new fracture repair technologies seek to serve the diverse unmet needs of patients and surgeons. Innovations in this space treat the entire fracture site, preventing infection and minimizing the need for surgery. We estimate that the global trauma market reached $8.3 billion in 2023. In this article, we highlight three companies...
A host of new fracture repair technologies seek to serve the diverse unmet needs of patients and surgeons. Innovations in this space treat the entire fracture site, preventing infection and minimizing the need for surgery. We estimate that the global trauma market reached $8.3 billion in 2023. In this article, we highlight three companies that seek to capture a portion of the market with novel approaches to fracture repair.

Endeavor Orthopaedics combines the plate, screws and suture into one kit, which allows surgeons to accommodate fracture fixation and soft tissue tasks with a single implant.
Endeavor Orthopaedics Focuses on Bone and Soft Tissue
Endeavor Orthopaedics has developed a patellar fixation solution that is the only product of its kind “with built-in soft tissue management provided in a single-use kit,” according to the company. By combining the plate, screws and suture into one kit, surgeons are able to accommodate fracture fixation and soft tissue tasks with a single implant.
The Summit Patella Plating System offers a biomechanical advantage during patient recovery by off-loading strain onto a single, interconnected construct. The device is FDA 510(k)-cleared and has been shown in clinical studies to lower reoperation rates and instances of the loosening and migration of hardware.
“Other device companies have patella plates on the market, but no one else has one that comes with a sterile and integrated suture,” said Endeavor Orthopaedics’ CEO Greg Parranto. “It’s a technology for any type of avulsion fracture where soft tissue management is needed to solve the repair.”
Inventor, surgeon and company Founder Brent Norris, M.D., was inspired to develop the device in 2011 after being unsatisfied with the uses and outcomes of other options on the market.
“By only treating the bony element of the injury with cannulated screws and orthopedic wire, we were not fully addressing the soft tissue component of the injury or, more importantly, the forces of the extensor mechanism,” Dr. Norris said. “Until these components are addressed, surgeons aren’t optimizing repairs.”
Patella fracture repair is a small market, but one that needs better solutions and can demonstrate the importance of treating bone and soft tissue issues at the fixation site, Mr. Parranto said. The company is raising Series B funding, which will be allocated to further develop its product pipeline and commercial model.

ABL Medical, a subsidiary of IlluminOss Medical, has shown that it’s proprietary multi-frequency illumination system can eliminate different species of bacteria.
IlluminOss Medical Advances Antimicrobial Device
Launched in 2018, IlluminOss’ Bone Stabilization System was recently shown to be adapted for antimicrobial applications. The platform is designed to prevent and treat pathological fractures of the humerus, radius and ulna caused by metastatic bone disease and fragility. It uses light-curable liquid monomer stored in an expandable catheter to stabilize the bone.
Data shared by ABL Medical, a subsidiary of IlluminOss Medical, confirms that its fiber-optic blue light tech can be tailored for antimicrobial use in common medical devices. The device’s proprietary multi-frequency illumination system can eliminate different species of bacteria by disrupting their oxygen-transportation molecules, which initiates a chain reaction that results in the breakdown of cells. Blue light impacts microbes so quickly and entirely that they don’t have time to develop resistance, like they can with conventional antibiotic treatments, according to ABL Medical.
The system, which ABL began developing in 2015, includes an optical light fiber that can emit blue light within a catheter or cannulated medical instrument to kill bacteria. This feature could potentially allow for the application of blue light for new and different orthopedic uses that may not have been previously possible. The system is installed through a small, minimally invasive percutaneous incision. After the implant is cured and polymerized, it uniquely conforms to a patient’s intramedullary canal.
Numerous patents have been issued for the ABL system. The company is collecting clinical data to present to FDA to determine the best regulatory pathway, said Robert Rabiner, Chief Technology Officer and Founder of IlluminOss.

PolyArmor is the first non-invasive external fixation system that’s specifically indicated for wrist fractures
Cambridge Orthopaedic Labs Takes Non-Invasive Approach
Conventional external fixators are fitted into bone with plates, rods and screws. Cambridge Orthopaedic Labs’ PolyArmor instead stabilizes fractures with a set of linkages that can be adjusted to fit a patient’s unique anatomy.
“PolyArmor is the first non-invasive external fixation system that’s specifically indicated for wrist fractures,” said David Anderson, CEO of Cambridge Orthopaedic Labs. “However, the technology has global application in fracture repair and can be used in other areas of the body.”
PolyArmor consists of three cuffs, which slip over the hand, the fractured area of the wrist and the upper forearm. The cuffs are connected by adjustable carbon fiber rods. The device is radiolucent, which allows physicians to set fractures under fluoroscopy or x-ray. The linkages and underlying padding hold reduced fractures in place for the duration of the healing.
Mr. Anderson said that the system is “infinitely adjustable” to the anatomy of the wrist and that the company’s single SKU can fit roughly 90% of the global population. “By adjusting the linkages, surgeons can apply the equivalent of three-point fixation to a fracture without performing surgery,” he added.
PolyArmor received FDA 510(k) clearance in 2022 and was launched in March 2023. The technology was developed by company Founder Ali Bajwa, M.D., F.R.C.S., who experimented with many different configurations before ultimately choosing a linkage system to manage fractures.
Dr. Bajwa wants to deliver surgery-free wrist fracture management to vulnerable populations “because most people who break their wrists are under the age of 14 and over the age of 60,” Mr. Anderson noted. The linkages and padding are also completely immersible and can be worn while patients shower and swim.
By healing simple and complex wrist fractures without surgery, PolyArmor also lessens physicians’ workloads and limits healthcare spending.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
PM
Patrick McGuire is an ORTHOWORLD Contributor.







