
 Copy to clipboard
Copy to clipboard 
NanoHive Medical announced the award of a Federal Supply Schedule Contract by the U.S. Department of Veteran Affairs (VA). The contract grants access to the Veteran’s Health Administration for the company’s complete line of 3D-printed Hive™ interbody devices that feature proprietary Soft Titanium® technology.
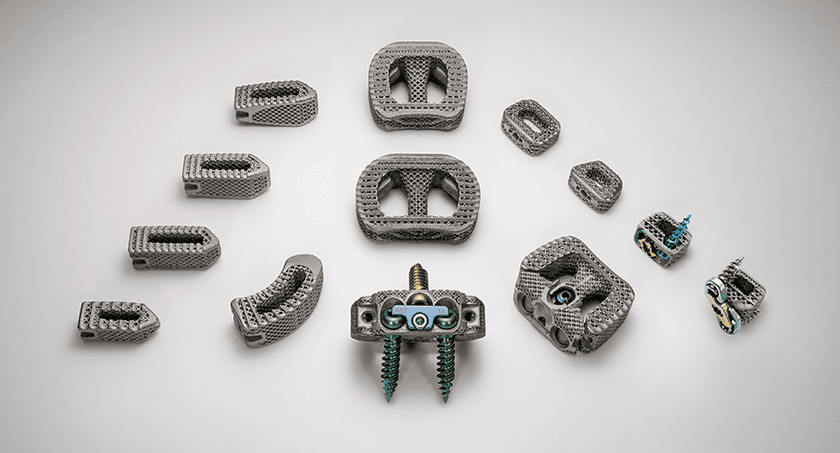
NanoHive Spinal Device Family
The VA is the largest healthcare system in the United States, serving over 9 million enrolled veterans, 6.1 million of which receive healthcare at over 150 hospitals in the U.S. There are 400,000 medical and surgical discharges annually at these facilities.
Hive™ Soft Titanium®, used in the company’s spinal interbody devices, combines 3D-printed titanium with a proprietary NanoHive® surface treatment. The structural lattice is designed to offer implant stiffness control and durability.
The company recently launched its FDA-cleared Hive™ Standalone Cervical System, as well as the Hive™ Standalone Anterior Lumbar System. NanoHive currently distributes their products through a network of independent distributors as well as two strategic partners, Accelus and Precision Spine.
Patrick O’Donnell, President & CEO of NanoHive Medical, said, “We are excited about the approval on the Federal Supply Schedule Contract and very proud to provide our unique technology to the VA System and our country’s veterans.”
Source: NanoHive Medical, LLC
NanoHive Medical announced the award of a Federal Supply Schedule Contract by the U.S. Department of Veteran Affairs (VA). The contract grants access to the Veteran’s Health Administration for the company’s complete line of 3D-printed Hive™ interbody devices that feature proprietary Soft Titanium® technology.
The VA is the largest...
NanoHive Medical announced the award of a Federal Supply Schedule Contract by the U.S. Department of Veteran Affairs (VA). The contract grants access to the Veteran’s Health Administration for the company’s complete line of 3D-printed Hive™ interbody devices that feature proprietary Soft Titanium® technology.

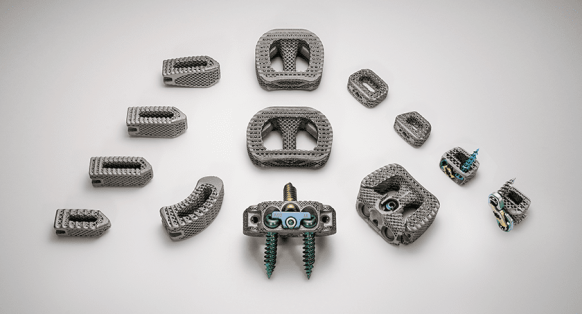
NanoHive Spinal Device Family
The VA is the largest healthcare system in the United States, serving over 9 million enrolled veterans, 6.1 million of which receive healthcare at over 150 hospitals in the U.S. There are 400,000 medical and surgical discharges annually at these facilities.
Hive™ Soft Titanium®, used in the company’s spinal interbody devices, combines 3D-printed titanium with a proprietary NanoHive® surface treatment. The structural lattice is designed to offer implant stiffness control and durability.
The company recently launched its FDA-cleared Hive™ Standalone Cervical System, as well as the Hive™ Standalone Anterior Lumbar System. NanoHive currently distributes their products through a network of independent distributors as well as two strategic partners, Accelus and Precision Spine.
Patrick O’Donnell, President & CEO of NanoHive Medical, said, “We are excited about the approval on the Federal Supply Schedule Contract and very proud to provide our unique technology to the VA System and our country’s veterans.”
Source: NanoHive Medical, LLC

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







