
 Copy to clipboard
Copy to clipboard 
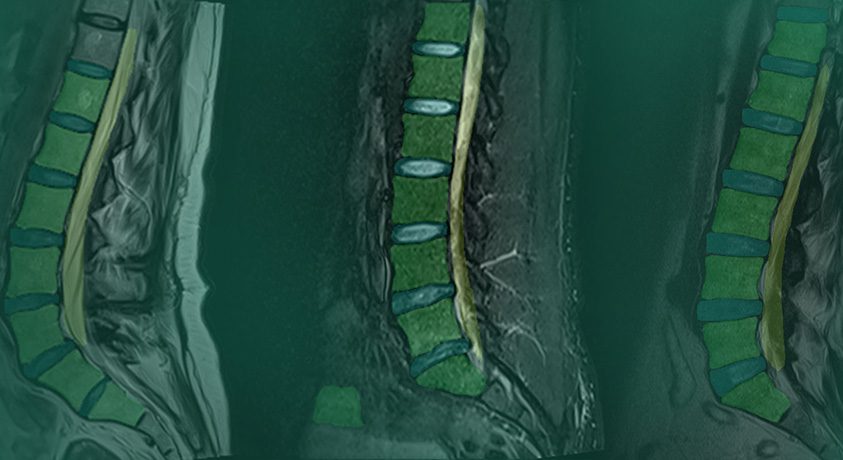
MSKai received FDA 510(k) clearance for lumbar spine MRI analysis.
MSKai is a tool that assists qualified medical professionals in the evaluation of previously acquired T2-weighted lumbar spine MRIs. The software enables users to perform anatomy segmentation, labeling, measurement and export of quantitative and qualitative results into customizable reports. By delivering objective, repeatable spine measurements, MSKai supports greater efficiency and consistency in clinical imaging workflows.
MSKai is not a diagnostic device and does not provide or recommend any medical diagnosis or treatment. Instead, it serves as a decision-support tool that offers clear, repeatable insights for users trained in medical imaging. Users are responsible for confirming preferences, verifying automated measurements, and finalizing reports in accordance with clinical best practices.
“FDA’s decision confirms that MSKai meets rigorous safety and performance standards as a spine imaging tool,” said Chip Wade, Ph. D., chief operating officer at MSKai. “We’re proud to deliver a product that gives healthcare professionals enhanced capabilities in lumbar spine analysis while reinforcing the central role of expert clinical judgement.”
Source: MSKai
MSKai received FDA 510(k) clearance for lumbar spine MRI analysis.
MSKai is a tool that assists qualified medical professionals in the evaluation of previously acquired T2-weighted lumbar spine MRIs. The software enables users to perform anatomy segmentation, labeling, measurement and export of quantitative and qualitative results into...
MSKai received FDA 510(k) clearance for lumbar spine MRI analysis.
MSKai is a tool that assists qualified medical professionals in the evaluation of previously acquired T2-weighted lumbar spine MRIs. The software enables users to perform anatomy segmentation, labeling, measurement and export of quantitative and qualitative results into customizable reports. By delivering objective, repeatable spine measurements, MSKai supports greater efficiency and consistency in clinical imaging workflows.
MSKai is not a diagnostic device and does not provide or recommend any medical diagnosis or treatment. Instead, it serves as a decision-support tool that offers clear, repeatable insights for users trained in medical imaging. Users are responsible for confirming preferences, verifying automated measurements, and finalizing reports in accordance with clinical best practices.
“FDA’s decision confirms that MSKai meets rigorous safety and performance standards as a spine imaging tool,” said Chip Wade, Ph. D., chief operating officer at MSKai. “We’re proud to deliver a product that gives healthcare professionals enhanced capabilities in lumbar spine analysis while reinforcing the central role of expert clinical judgement.”
Source: MSKai

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







