
 Copy to clipboard
Copy to clipboard 
Mortise Medical received FDA 510(k) clearance to market the SyndesMetrics’ Syndesmosis Repair system.
SyndesMetrics is designed to support predictable/repeatable anatomic reduction, restoration of physiological motion and restoration of ankle stability.
The SyndesMetrics Reduction Clamp features anatomic-referencing clamp points, surgeon-controlled calibrated clamping force and an integrated drill guide. The Reduction Clamp and implant are compatible with distal fibula plating systems. The implant has minimal hardware prominence, no suture prominence and a reversible locking mechanism to secure suture tape that connects the tibial and fibular components.
Mortise Medical is a medical device startup incubated and operated by Surgical Frontiers.
Source: Mortise Medical
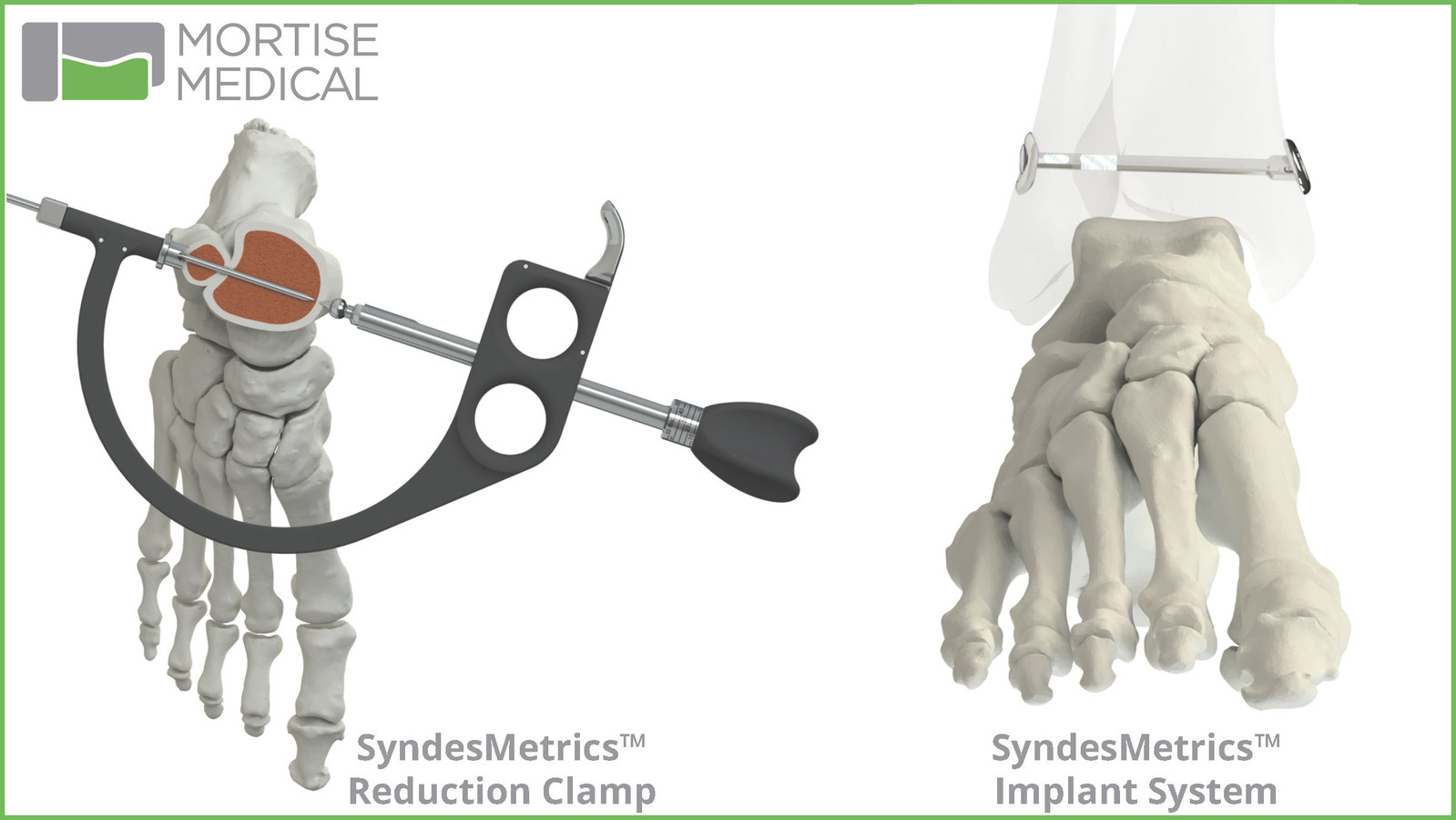
Image courtesy of Mortise Medical
Mortise Medical received FDA 510(k) clearance to market the SyndesMetrics' Syndesmosis Repair system.
SyndesMetrics is designed to support predictable/repeatable anatomic reduction, restoration of physiological motion and restoration of ankle stability.
The SyndesMetrics Reduction Clamp features anatomic-referencing clamp...
Mortise Medical received FDA 510(k) clearance to market the SyndesMetrics’ Syndesmosis Repair system.
SyndesMetrics is designed to support predictable/repeatable anatomic reduction, restoration of physiological motion and restoration of ankle stability.
The SyndesMetrics Reduction Clamp features anatomic-referencing clamp points, surgeon-controlled calibrated clamping force and an integrated drill guide. The Reduction Clamp and implant are compatible with distal fibula plating systems. The implant has minimal hardware prominence, no suture prominence and a reversible locking mechanism to secure suture tape that connects the tibial and fibular components.
Mortise Medical is a medical device startup incubated and operated by Surgical Frontiers.
Source: Mortise Medical

Image courtesy of Mortise Medical

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







