
 Copy to clipboard
Copy to clipboard 
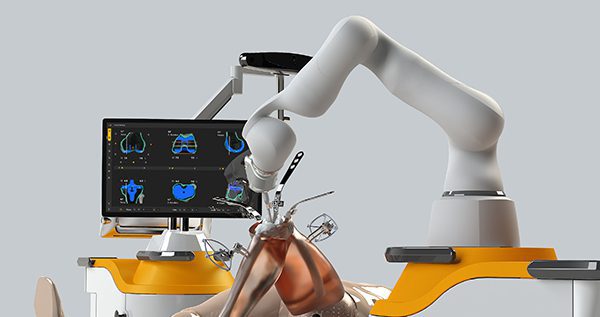
Monogram Orthopaedics executed a conditional purchase order to initiate a pilot program with a global distributor.
Monogram will update its system software to facilitate robotic surgery with the distributor’s implants for comprehensive evaluation. Notably, the company anticipates that testing may include robotic surgeries on live patients. The distributor will conduct its evaluation outside of the United States.
As Monogram continues to pursue a 510(k) clearance in the United States, the Company anticipates that this development could help bolster those efforts. The Company anticipates that following a successful evaluation, an Outside the United States (OUS) commercialization strategy could also help establish the commercial viability of the system and provide clinical data helpful for a US regulatory submission.
“The FDA has well-established guidelines for companies to leverage clinical data obtained outside the United States for 510(k) submissions,” said Dave McGurl, Vice President of Regulatory Affairs at MCRA, a leading medical device Clinical Research Organization (CRO) and advisory firm. “Collecting live patient data internationally, especially if there is commercial interest for the underlying product, can accelerate the time to launch domestically, reduce the costs of doing so, and greatly mitigate the risks of a failed submission – this could be a favorable development for Monogram.”
Monogram is currently performing testing at its Austin facility and expects to ship the first system in 4Q23, provided developments proceed as expected. The conditional purchase order provides for the sale of the company’s surgical robot system to an international healthcare provider and shipment to be made by November 30, 2023. The provider reserves the right to reject or cancel the order.
Monogram seeks to demo the next generation of its product pipeline later this year.
“This purchase order marks a pivotal moment for Monogram as we continue executing our strategic roadmap,” said Ben Sexson, Chief Executive Officer of Monogram Orthopaedics. “Our ability to complete the system evaluation successfully is a testament to our team’s dedication and the market potential for our current and next-generation product pipelines. This significant milestone on our journey toward commercialization brings us many steps closer to adoption of our technology in the operating room while providing an upfront source of revenue and cash flow.”
Source: Monogram Orthopaedics
Monogram Orthopaedics executed a conditional purchase order to initiate a pilot program with a global distributor.
Monogram will update its system software to facilitate robotic surgery with the distributor’s implants for comprehensive evaluation. Notably, the company anticipates that testing may include robotic surgeries on live patients. The...
Monogram Orthopaedics executed a conditional purchase order to initiate a pilot program with a global distributor.
Monogram will update its system software to facilitate robotic surgery with the distributor’s implants for comprehensive evaluation. Notably, the company anticipates that testing may include robotic surgeries on live patients. The distributor will conduct its evaluation outside of the United States.
As Monogram continues to pursue a 510(k) clearance in the United States, the Company anticipates that this development could help bolster those efforts. The Company anticipates that following a successful evaluation, an Outside the United States (OUS) commercialization strategy could also help establish the commercial viability of the system and provide clinical data helpful for a US regulatory submission.
“The FDA has well-established guidelines for companies to leverage clinical data obtained outside the United States for 510(k) submissions,” said Dave McGurl, Vice President of Regulatory Affairs at MCRA, a leading medical device Clinical Research Organization (CRO) and advisory firm. “Collecting live patient data internationally, especially if there is commercial interest for the underlying product, can accelerate the time to launch domestically, reduce the costs of doing so, and greatly mitigate the risks of a failed submission – this could be a favorable development for Monogram.”
Monogram is currently performing testing at its Austin facility and expects to ship the first system in 4Q23, provided developments proceed as expected. The conditional purchase order provides for the sale of the company’s surgical robot system to an international healthcare provider and shipment to be made by November 30, 2023. The provider reserves the right to reject or cancel the order.
Monogram seeks to demo the next generation of its product pipeline later this year.
“This purchase order marks a pivotal moment for Monogram as we continue executing our strategic roadmap,” said Ben Sexson, Chief Executive Officer of Monogram Orthopaedics. “Our ability to complete the system evaluation successfully is a testament to our team’s dedication and the market potential for our current and next-generation product pipelines. This significant milestone on our journey toward commercialization brings us many steps closer to adoption of our technology in the operating room while providing an upfront source of revenue and cash flow.”
Source: Monogram Orthopaedics

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







