
 Copy to clipboard
Copy to clipboard 
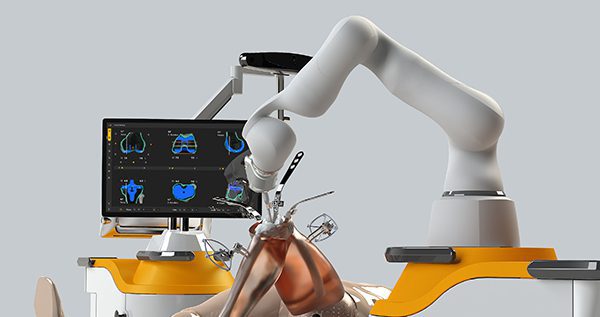
Monogram Orthopedics seeks to personalize joint replacement through a combination of innovative implants and robotics. Still early in its quest, the company recently announced it’s raising $34 million in a Series B round of funding.
The capital will be deployed to launch its implant in the first half of 2022, build out its sales channel and support further growth. The Austin, Texas-headquartered company will enter verification and validation for its robot in 2022 to launch it in 2023.
We spoke to Monogram Orthopedics Co-Founder and CEO Ben Sexson about the company’s plans and what trends he sees in the orthopedic market.
How would you describe today’s funding environment?
Mr. Sexson: Fundraising large budget development projects for pre-FDA-approved medtech devices, especially orthopedic devices, is a purgatory. There is an absolute scarcity of capital, especially for ortho startups. Funds that previously supported pre-FDA-approved developments have become increasingly defensive and more focused on biotech; large strategics rarely deploy meaningful early-stage capital. The seed and angel capital groups generally do not deploy sufficient money to hire teams and get development projects off the ground to significant traction. It’s a very challenging environment!
You performed your first total knee procedure in 2021 and are now a revenue-generating company. What is your strategy moving forward? Can you share what’s in your product pipeline?
Mr. Sexson: We entered into a rather complex deal to secure long-term supply pricing of various implant components at levels we think will be favorable to our company long-term. As part of those negotiations, we placed POs for inventory we sold, but we do not intend to continue marketing those products as-is.
We are launching our first product in 1H22 and will be showing it to surgeons at AAOS. Our first product will be a press-fit total knee that we anticipate could be competitive with the current state of the art.
Monogram expects to disrupt orthopedics with robotics and additively manufactured implants. How is your approach different? When do you expect to reach the market with these technologies?
Mr. Sexson: We are commercializing via a multi-generational product release strategy. Our first-gen Monogram implant will be a press-fit total knee that we plan to launch in 1H22. We plan to submit our robot for FDA approval in 2023.
We believe that Monogram can make significant improvements to the current state of the art in ortho-robotics. On the implant side, we have entirely rethought implant design from the outside-in. We asked ourselves, if you have a CT scan and a cutting navigated robot, what is the optimal shape for an implant? Our goal is to address loosening, stress-shielding, and ease of revision concerns with what we call our patient-optimized designs.
What are some of the primary trends you’re seeing in joint replacement today?
Mr. Sexson: As you know, the shift to cementless knees is one of the fastest-growing areas in recon. The cementless knee segment is projected to grow by an estimated 400,000 procedures from 2020 to 2024 ($1.21 billion). The beautiful thing is that ASPs on cementless knees are also higher (about 10% higher). Cementless TKAs accounted for 42% of Stryker’s TKA volume in 2020, up from 30% in 2018. The new cementless Attune is one of J&J’s fastest-growing products. This is where the market is quickly moving, and we hope to have a best-in-class product to address this growing opportunity.
We believe a current obvious greenfield opportunity is a robotic press-fit partial knee. We think it’s remarkable nobody has commercialized this yet (we have licensed an FDA-approved partial knee, and it’s in our pipeline).
We consider a robotic hip that prepares the stem an open opportunity, but sadly we haven’t seen the market enthusiasm on the hip side. Maybe that could change with time and hardware advances.
As Mako utilization continues to march on, we see more surgeons questioning strict adherence to conventional balancing approaches (for example, measured resection). We think the next level of robotics will be figuring out how to use data to make balancing soft tissue flawless and fiddle-factor-free. We certainly believe the industry will be rethinking alignment approaches over the next five to ten years.
We expect continued headwinds for robot pricing, and we hope to apply pressure on robot surgical times.
Monogram Orthopedics seeks to personalize joint replacement through a combination of innovative implants and robotics. Still early in its quest, the company recently announced it's raising $34 million in a Series B round of funding.
The capital will be deployed to launch its implant in the first half of 2022, build out its sales channel and...
Monogram Orthopedics seeks to personalize joint replacement through a combination of innovative implants and robotics. Still early in its quest, the company recently announced it’s raising $34 million in a Series B round of funding.
The capital will be deployed to launch its implant in the first half of 2022, build out its sales channel and support further growth. The Austin, Texas-headquartered company will enter verification and validation for its robot in 2022 to launch it in 2023.
We spoke to Monogram Orthopedics Co-Founder and CEO Ben Sexson about the company’s plans and what trends he sees in the orthopedic market.
How would you describe today’s funding environment?
Mr. Sexson: Fundraising large budget development projects for pre-FDA-approved medtech devices, especially orthopedic devices, is a purgatory. There is an absolute scarcity of capital, especially for ortho startups. Funds that previously supported pre-FDA-approved developments have become increasingly defensive and more focused on biotech; large strategics rarely deploy meaningful early-stage capital. The seed and angel capital groups generally do not deploy sufficient money to hire teams and get development projects off the ground to significant traction. It’s a very challenging environment!
You performed your first total knee procedure in 2021 and are now a revenue-generating company. What is your strategy moving forward? Can you share what’s in your product pipeline?
Mr. Sexson: We entered into a rather complex deal to secure long-term supply pricing of various implant components at levels we think will be favorable to our company long-term. As part of those negotiations, we placed POs for inventory we sold, but we do not intend to continue marketing those products as-is.
We are launching our first product in 1H22 and will be showing it to surgeons at AAOS. Our first product will be a press-fit total knee that we anticipate could be competitive with the current state of the art.
Monogram expects to disrupt orthopedics with robotics and additively manufactured implants. How is your approach different? When do you expect to reach the market with these technologies?
Mr. Sexson: We are commercializing via a multi-generational product release strategy. Our first-gen Monogram implant will be a press-fit total knee that we plan to launch in 1H22. We plan to submit our robot for FDA approval in 2023.
We believe that Monogram can make significant improvements to the current state of the art in ortho-robotics. On the implant side, we have entirely rethought implant design from the outside-in. We asked ourselves, if you have a CT scan and a cutting navigated robot, what is the optimal shape for an implant? Our goal is to address loosening, stress-shielding, and ease of revision concerns with what we call our patient-optimized designs.
What are some of the primary trends you’re seeing in joint replacement today?
Mr. Sexson: As you know, the shift to cementless knees is one of the fastest-growing areas in recon. The cementless knee segment is projected to grow by an estimated 400,000 procedures from 2020 to 2024 ($1.21 billion). The beautiful thing is that ASPs on cementless knees are also higher (about 10% higher). Cementless TKAs accounted for 42% of Stryker’s TKA volume in 2020, up from 30% in 2018. The new cementless Attune is one of J&J’s fastest-growing products. This is where the market is quickly moving, and we hope to have a best-in-class product to address this growing opportunity.
We believe a current obvious greenfield opportunity is a robotic press-fit partial knee. We think it’s remarkable nobody has commercialized this yet (we have licensed an FDA-approved partial knee, and it’s in our pipeline).
We consider a robotic hip that prepares the stem an open opportunity, but sadly we haven’t seen the market enthusiasm on the hip side. Maybe that could change with time and hardware advances.
As Mako utilization continues to march on, we see more surgeons questioning strict adherence to conventional balancing approaches (for example, measured resection). We think the next level of robotics will be figuring out how to use data to make balancing soft tissue flawless and fiddle-factor-free. We certainly believe the industry will be rethinking alignment approaches over the next five to ten years.
We expect continued headwinds for robot pricing, and we hope to apply pressure on robot surgical times.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







