
 Copy to clipboard
Copy to clipboard 
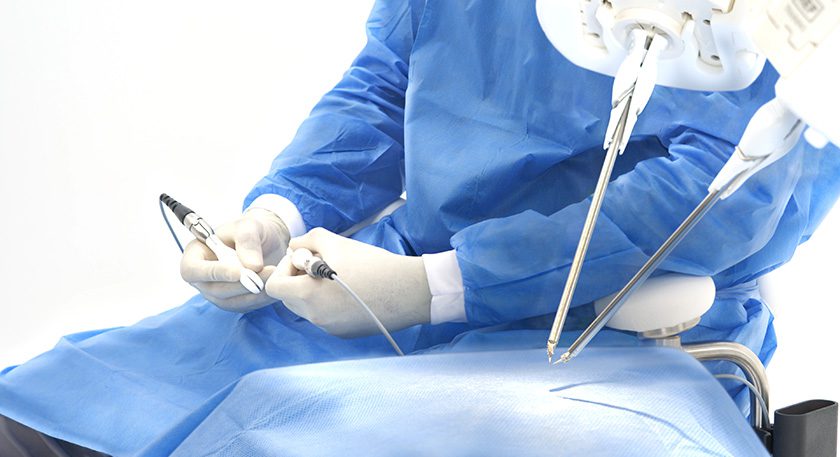
MMI (Medical Microinstruments, Inc.) marked completion of its first clinical cases in the U.S. using the Symani Surgical System.
Both robotic-assisted procedures were reconstructive extremity microsurgeries. In one case, the team performed a “free bone transfer” procedure on a patient who suffered a traumatic injury; the procedure involved transferring a segment of bone and skin from their leg to a damaged bone in their forearm. The surgical team revascularized the bone segment using the microsurgical robot to reconnect the tiny blood vessels and facilitate successful transfer. The second case involved a patient at risk for leg amputation due to an infected knee prosthesis with soft tissue deficiency. The team repaired the severe knee wound with muscle and skin from the patient’s back and robotically reconnected the blood vessels to promote revascularization.
FDA has granted De Novo Classification to the Symani Surgical System, making it the only commercially available platform in the U.S. for reconstructive microsurgery. It addresses the scale and complexities of microsurgery and supermicrosurgery such as the anastomosis and suturing of small anatomical structures – like blood and lymphatic vessels – during open surgical procedures. By allowing surgeons to replicate the natural movements of the human hand at the micro scale, it can expand treatment options for patients in need of advanced surgical techniques. It is designed to help restore quality of life for more patients, accelerate the number of surgeons able to push the boundaries of complex procedures for delicate anatomy and enable hospitals to expand their open surgical programs.
In early 2024, MMI announced a raise of $110 million in Series C financing to support commercialization of Symani in high-growth markets and continued investment in studies. The company has raised over $200 million in funding to date.
“The first U.S. cases are a paramount milestone in the global expansion of the Symani Surgical System, and we’re honored to have been able to work with Penn Medicine’s premiere orthopedic and plastic surgery departments to achieve it,” said Mark Toland, CEO at MMI. “Today marks the beginning of a new era in surgical innovation, as patients across the country with conditions that require complex microsurgical techniques, such as extremity reconstruction, autologous breast reconstruction post cancer resection and lymphedema repair, will now have expanded access to treatment options.”
Source: MMI
MMI (Medical Microinstruments, Inc.) marked completion of its first clinical cases in the U.S. using the Symani Surgical System.
Both robotic-assisted procedures were reconstructive extremity microsurgeries. In one case, the team performed a “free bone transfer” procedure on a patient who suffered a traumatic injury; the procedure involved...
MMI (Medical Microinstruments, Inc.) marked completion of its first clinical cases in the U.S. using the Symani Surgical System.
Both robotic-assisted procedures were reconstructive extremity microsurgeries. In one case, the team performed a “free bone transfer” procedure on a patient who suffered a traumatic injury; the procedure involved transferring a segment of bone and skin from their leg to a damaged bone in their forearm. The surgical team revascularized the bone segment using the microsurgical robot to reconnect the tiny blood vessels and facilitate successful transfer. The second case involved a patient at risk for leg amputation due to an infected knee prosthesis with soft tissue deficiency. The team repaired the severe knee wound with muscle and skin from the patient’s back and robotically reconnected the blood vessels to promote revascularization.
FDA has granted De Novo Classification to the Symani Surgical System, making it the only commercially available platform in the U.S. for reconstructive microsurgery. It addresses the scale and complexities of microsurgery and supermicrosurgery such as the anastomosis and suturing of small anatomical structures – like blood and lymphatic vessels – during open surgical procedures. By allowing surgeons to replicate the natural movements of the human hand at the micro scale, it can expand treatment options for patients in need of advanced surgical techniques. It is designed to help restore quality of life for more patients, accelerate the number of surgeons able to push the boundaries of complex procedures for delicate anatomy and enable hospitals to expand their open surgical programs.
In early 2024, MMI announced a raise of $110 million in Series C financing to support commercialization of Symani in high-growth markets and continued investment in studies. The company has raised over $200 million in funding to date.
“The first U.S. cases are a paramount milestone in the global expansion of the Symani Surgical System, and we’re honored to have been able to work with Penn Medicine’s premiere orthopedic and plastic surgery departments to achieve it,” said Mark Toland, CEO at MMI. “Today marks the beginning of a new era in surgical innovation, as patients across the country with conditions that require complex microsurgical techniques, such as extremity reconstruction, autologous breast reconstruction post cancer resection and lymphedema repair, will now have expanded access to treatment options.”
Source: MMI

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







