
 Copy to clipboard
Copy to clipboard 
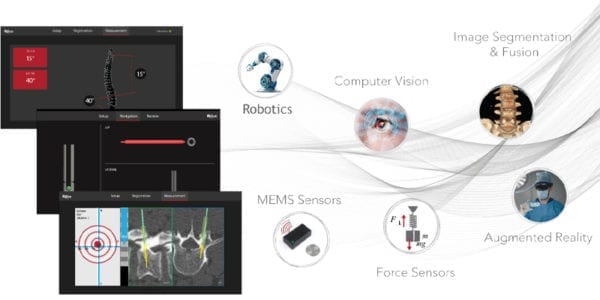
MiRus was granted FDA 510(k) clearance to market the GALILEO™ Spine Alignment Monitoring System, a non-optical, wireless, real-time measurement system for segmental and global sagittal spine alignment.
GALILEO is reportedly the first FDA-cleared product that can intra-operatively measure sagittal plane alignment without the need for repeated imaging. MiRus plans to incorporate dynamic tracking and feedback into its robotics platform.
Source: MiRus, LLC
MiRus was granted FDA 510(k) clearance to market the GALILEO™ Spine Alignment Monitoring System, a non-optical, wireless, real-time measurement system for segmental and global sagittal spine alignment.
GALILEO is reportedly the first FDA-cleared product that can intra-operatively measure sagittal plane alignment without the need for...
MiRus was granted FDA 510(k) clearance to market the GALILEO™ Spine Alignment Monitoring System, a non-optical, wireless, real-time measurement system for segmental and global sagittal spine alignment.
GALILEO is reportedly the first FDA-cleared product that can intra-operatively measure sagittal plane alignment without the need for repeated imaging. MiRus plans to incorporate dynamic tracking and feedback into its robotics platform.
Source: MiRus, LLC

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.