
 Copy to clipboard
Copy to clipboard 
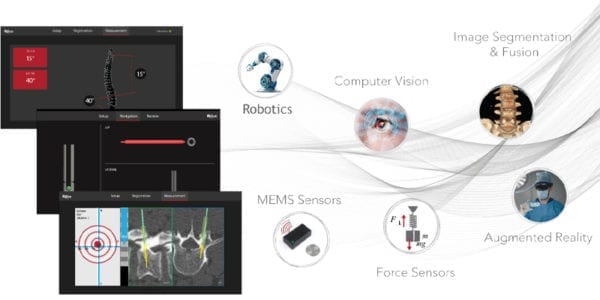
MiRus was granted FDA 510(k) clearance to market the GALILEO™ Spine Alignment Monitoring System, a non-optical, wireless, real-time measurement system for segmental and global sagittal spine alignment.
GALILEO is reportedly the first FDA-cleared product that can intra-operatively measure sagittal plane alignment without the need for repeated imaging. MiRus plans to incorporate dynamic tracking and feedback into its robotics platform.
Source: MiRus, LLC
MiRus was granted FDA 510(k) clearance to market the GALILEO™ Spine Alignment Monitoring System, a non-optical, wireless, real-time measurement system for segmental and global sagittal spine alignment.
GALILEO is reportedly the first FDA-cleared product that can intra-operatively measure sagittal plane alignment without the need for...
MiRus was granted FDA 510(k) clearance to market the GALILEO™ Spine Alignment Monitoring System, a non-optical, wireless, real-time measurement system for segmental and global sagittal spine alignment.
GALILEO is reportedly the first FDA-cleared product that can intra-operatively measure sagittal plane alignment without the need for repeated imaging. MiRus plans to incorporate dynamic tracking and feedback into its robotics platform.
Source: MiRus, LLC

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







