
 Copy to clipboard
Copy to clipboard 
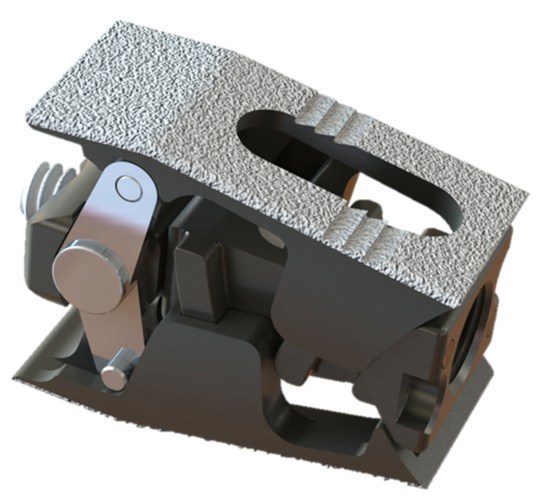
MiRus received FDA 510(k) clearance to market its IO™ Expandable Lumbar Interbody.
The device can be used in both PLIF and TLIF procedures. It features the lowest insertion profile (4mm) and greatest expansion (18mm) and lordosis (24◦) in a single implant of any expandable interbody on the market. In addition to the one-step expansion, the implant allows for 2.5cc of biologic to be post-packed in the intervertebral space.
Powered by the MoRe® expansion engine, the IO Expandable Lumbar Interbody allows minimization of insertion height and maximization of expansion height, lordosis thus making it easier for surgeons to restore lordosis, maintain sagittal balance while reducing the challenges of insertion and related neural injury.
FDA 510(k) clearance allows the IO™ Expandable Lumbar Interbody to be used in skeletally mature patients with Degenerative Disc Disease (DDD) at one or two contiguous levels from L1-L2 to L5-S1.
Source: MiRus
MiRus received FDA 510(k) clearance to market its IO™ Expandable Lumbar Interbody.
The device can be used in both PLIF and TLIF procedures. It features the lowest insertion profile (4mm) and greatest expansion (18mm) and lordosis (24◦) in a single implant of any expandable interbody on the market. In addition to the one-step expansion, the...
MiRus received FDA 510(k) clearance to market its IO™ Expandable Lumbar Interbody.
The device can be used in both PLIF and TLIF procedures. It features the lowest insertion profile (4mm) and greatest expansion (18mm) and lordosis (24◦) in a single implant of any expandable interbody on the market. In addition to the one-step expansion, the implant allows for 2.5cc of biologic to be post-packed in the intervertebral space.
Powered by the MoRe® expansion engine, the IO Expandable Lumbar Interbody allows minimization of insertion height and maximization of expansion height, lordosis thus making it easier for surgeons to restore lordosis, maintain sagittal balance while reducing the challenges of insertion and related neural injury.
FDA 510(k) clearance allows the IO™ Expandable Lumbar Interbody to be used in skeletally mature patients with Degenerative Disc Disease (DDD) at one or two contiguous levels from L1-L2 to L5-S1.
Source: MiRus

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







