
 Copy to clipboard
Copy to clipboard 
Since its formation as a wholly independent company in late 2020, Spine Innovations has continued to expand sales of its ESP spinal disc replacements, with more than 20,000 prostheses successfully implanted in patients around the world.
The company is now active in 17 countries, including new markets within Europe that opened in 2021. Spine Innovations will expand into Central and South America, including Mexico and Brazil, as well as additional European countries, including Norway and the Netherlands. The company is also pursuing FDA clearance to enter the U.S. market during 2022.
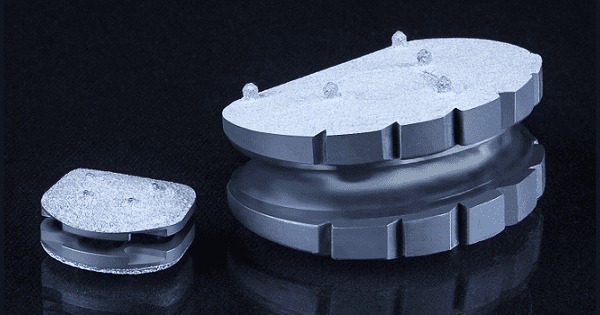
The company’s Cervical Prosthesis (CP-ESP®) and Lumbar Prosthesis (LP-ESP®) utilize a viscoelastic design to mimic the function and range of movements of a natural disc and allow the spine to behave naturally.
ESP discs combine two titanium endplates with an elastomeric cushion made of polycarbonate urethane. This structure is designed to imitate the natural disc, in which bony segments are connected by a spongy disc that provides flexibility while withstanding the pressures of compression and torsion. Short spikes, a rough outer surface and a coating of hydroxyapatite (HA) on the endplates are intended to help ensure stability and bony fixation of the implant over time.
Source: Spine Innovations
Since its formation as a wholly independent company in late 2020, Spine Innovations has continued to expand sales of its ESP spinal disc replacements, with more than 20,000 prostheses successfully implanted in patients around the world.
The company is now active in 17 countries, including new markets within Europe that opened in 2021. Spine...
Since its formation as a wholly independent company in late 2020, Spine Innovations has continued to expand sales of its ESP spinal disc replacements, with more than 20,000 prostheses successfully implanted in patients around the world.
The company is now active in 17 countries, including new markets within Europe that opened in 2021. Spine Innovations will expand into Central and South America, including Mexico and Brazil, as well as additional European countries, including Norway and the Netherlands. The company is also pursuing FDA clearance to enter the U.S. market during 2022.
The company’s Cervical Prosthesis (CP-ESP®) and Lumbar Prosthesis (LP-ESP®) utilize a viscoelastic design to mimic the function and range of movements of a natural disc and allow the spine to behave naturally.
ESP discs combine two titanium endplates with an elastomeric cushion made of polycarbonate urethane. This structure is designed to imitate the natural disc, in which bony segments are connected by a spongy disc that provides flexibility while withstanding the pressures of compression and torsion. Short spikes, a rough outer surface and a coating of hydroxyapatite (HA) on the endplates are intended to help ensure stability and bony fixation of the implant over time.
Source: Spine Innovations

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







