
 Copy to clipboard
Copy to clipboard 
Miach Orthopaedics initiated the Bridge Registry postmarket study of the company’s Bridge-Enhanced ACL Restoration (BEAR) Implant for anterior cruciate ligament (ACL) tears.
The Bridge Registry (NCT05398341) will assess real-world evidence of the BEAR Implant, with approval to enroll up to 750 patients at up to 30 sites in the U.S. Primary outcomes being tracked include knee function and feeling measured by International Knee Documentation Committee Subjective Knee Evaluation at two years and knee laxity measured with Lachman scoring at one year.
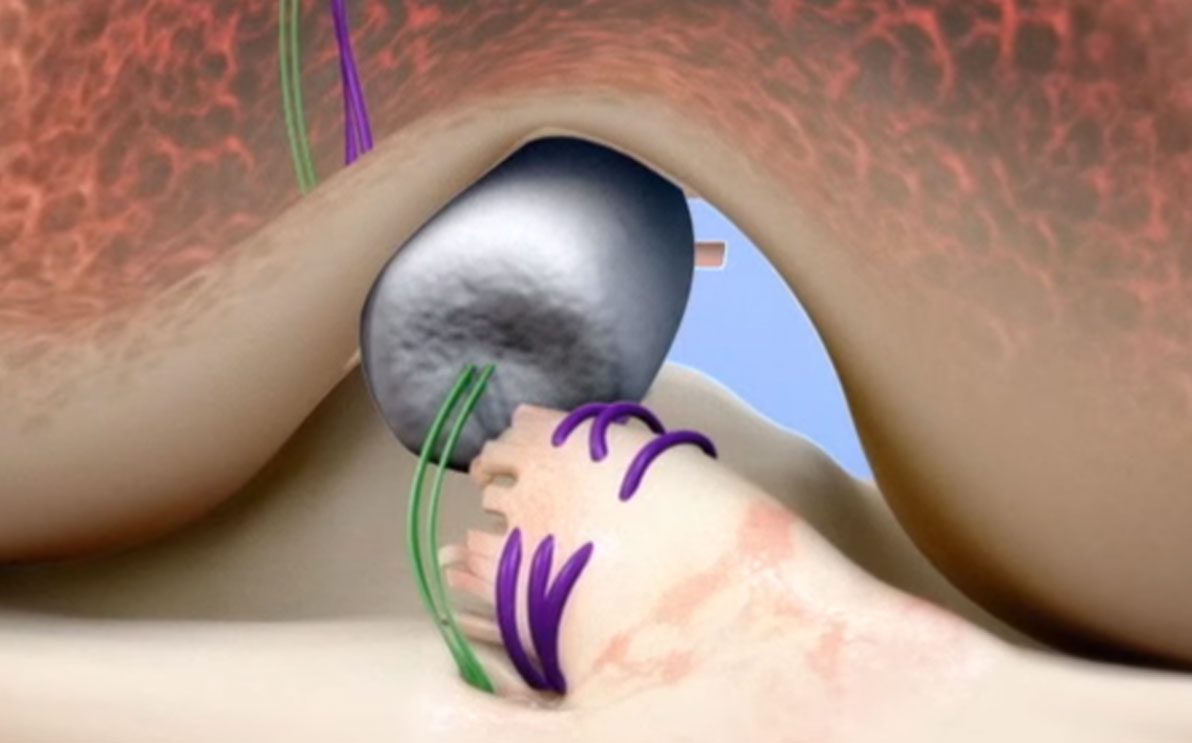
BEAR serves as a bridge to restore the patient’s own ACL and is reported to be the first medical technology that has been clinically proven to enable healing of a torn ACL. It is a paradigm shift from the current standard of care – reconstruction that replaces the ACL with a graft. The implant acts as a bridge to help the torn ends of the ACL heal together. It is designed to hold and protect the patient’s own blood in the gap between the ACL ends to allow the formation of a clot, which is necessary for healing.
The BEAR Implant was granted De Novo Approval from FDA in late 2020. It is indicated for skeletally mature patients at least 14 years of age with a complete rupture of the ACL, as confirmed by MRI. Patients must have an ACL stump attached to the tibia to facilitate the restoration.
Source: Miach Orthopaedics, Inc.
Miach Orthopaedics initiated the Bridge Registry postmarket study of the company’s Bridge-Enhanced ACL Restoration (BEAR) Implant for anterior cruciate ligament (ACL) tears.
The Bridge Registry (NCT05398341) will assess real-world evidence of the BEAR Implant, with approval to enroll up to 750 patients at up to 30 sites in the U.S. Primary...
Miach Orthopaedics initiated the Bridge Registry postmarket study of the company’s Bridge-Enhanced ACL Restoration (BEAR) Implant for anterior cruciate ligament (ACL) tears.
The Bridge Registry (NCT05398341) will assess real-world evidence of the BEAR Implant, with approval to enroll up to 750 patients at up to 30 sites in the U.S. Primary outcomes being tracked include knee function and feeling measured by International Knee Documentation Committee Subjective Knee Evaluation at two years and knee laxity measured with Lachman scoring at one year.
BEAR serves as a bridge to restore the patient’s own ACL and is reported to be the first medical technology that has been clinically proven to enable healing of a torn ACL. It is a paradigm shift from the current standard of care – reconstruction that replaces the ACL with a graft. The implant acts as a bridge to help the torn ends of the ACL heal together. It is designed to hold and protect the patient’s own blood in the gap between the ACL ends to allow the formation of a clot, which is necessary for healing.
The BEAR Implant was granted De Novo Approval from FDA in late 2020. It is indicated for skeletally mature patients at least 14 years of age with a complete rupture of the ACL, as confirmed by MRI. Patients must have an ACL stump attached to the tibia to facilitate the restoration.
Source: Miach Orthopaedics, Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







