
 Copy to clipboard
Copy to clipboard 
The first patient has been treated in Miach Orthopaedics’ BEAR III study of the BEAR® Implant, the first medical technology to clinically demonstrate that it enables healing of a patient’s torn anterior cruciate ligament (ACL). Miach initiated BEAR III to continue to build upon the extensive base of evidence for the BEAR Implant, which recently received De Novo approval from FDA.
BEAR III is a single-arm, multicenter cohort study enrolling 250 patients, all of whom will receive the BEAR Implant and will be followed for 10 years.
The BEAR III Trial for Bridge-Enhanced ACL Restoration is or will soon be enrolling patients at orthopedic centers in Georgia, Louisiana, Maryland, Massachusetts, New Jersey, Rhode Island and South Dakota.
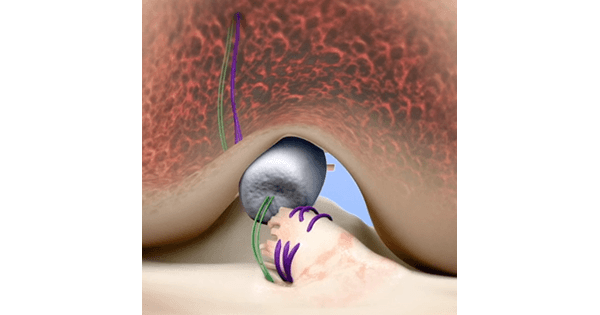
The Bridge-Enhanced® ACL Restoration (BEAR®) Implant is a proprietary bio-engineered implant used to facilitate healing of the torn ACL. Unlike reconstruction, which is the current standard of care, the BEAR Implant does not require a second surgical wound site to remove a healthy tendon from another part of the leg or the use of a donor tendon. The BEAR Implant acts as a bridge between the two ends of the torn ACL. The surgeon injects a small amount of the patient’s own blood into the implant and inserts it between the torn ends of the ACL in a minimally invasive procedure. The combination of the BEAR Implant and the patient’s blood enables the body to heal the torn ends of the ACL back together while maintaining the ACL’s original attachments to the femur and tibia. The BEAR Implant is absorbed by the body as the ACL heals.
Source: Miach Orthopaedics, Inc.
The first patient has been treated in Miach Orthopaedics' BEAR III study of the BEAR® Implant, the first medical technology to clinically demonstrate that it enables healing of a patient's torn anterior cruciate ligament (ACL). Miach initiated BEAR III to continue to build upon the extensive base of evidence for the BEAR Implant, which recently...
The first patient has been treated in Miach Orthopaedics’ BEAR III study of the BEAR® Implant, the first medical technology to clinically demonstrate that it enables healing of a patient’s torn anterior cruciate ligament (ACL). Miach initiated BEAR III to continue to build upon the extensive base of evidence for the BEAR Implant, which recently received De Novo approval from FDA.
BEAR III is a single-arm, multicenter cohort study enrolling 250 patients, all of whom will receive the BEAR Implant and will be followed for 10 years.
The BEAR III Trial for Bridge-Enhanced ACL Restoration is or will soon be enrolling patients at orthopedic centers in Georgia, Louisiana, Maryland, Massachusetts, New Jersey, Rhode Island and South Dakota.
The Bridge-Enhanced® ACL Restoration (BEAR®) Implant is a proprietary bio-engineered implant used to facilitate healing of the torn ACL. Unlike reconstruction, which is the current standard of care, the BEAR Implant does not require a second surgical wound site to remove a healthy tendon from another part of the leg or the use of a donor tendon. The BEAR Implant acts as a bridge between the two ends of the torn ACL. The surgeon injects a small amount of the patient’s own blood into the implant and inserts it between the torn ends of the ACL in a minimally invasive procedure. The combination of the BEAR Implant and the patient’s blood enables the body to heal the torn ends of the ACL back together while maintaining the ACL’s original attachments to the femur and tibia. The BEAR Implant is absorbed by the body as the ACL heals.
Source: Miach Orthopaedics, Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







