
 Copy to clipboard
Copy to clipboard 
Miach Orthopaedics gained FDA De Novo approval for the bioengineered Bridge-Enhanced® ACL Repair (BEAR®) Implant for the treatment of anterior cruciate ligament (ACL) tears. Limited market release is slated for early 2021.
BEAR is the first medical technology to clinically demonstrate that it enables healing/restoration of a torn ACL. This approach presents an alternative to reconstruction that replaces the ACL with a graft, and is the first new treatment for ACL tears in more than 30 years.
BEAR is designed to restore natural anatomy and function of the knee, and does not require a second surgical procedure to remove a healthy tendon from another part of the leg or use of a deceased donor’s tendon that requires special storage and handling requirements.
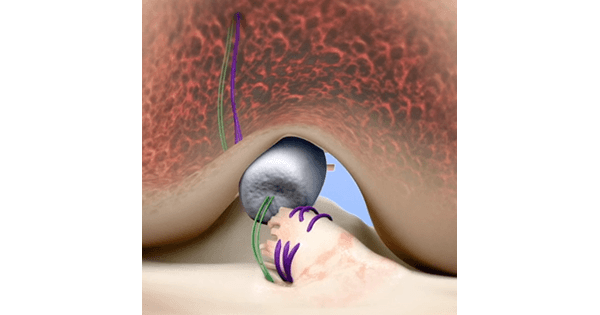
The resorbable implant is made from bovine collagen and secured via suture to bridge the gap between the torn ends of a patient’s ACL. The patient’s own blood is injected into the implant to form a device-protected clot that enables the body’s healing process. Within about eight weeks the implant is absorbed and replaced by the body’s own tissue.Pre-clinical studies suggest that the BEAR Implant may reduce the rate of osteoarthritis, estimated to occur in 78% of people with ACL tears, but this has not yet been confirmed in human trials.
“Preserving a patient’s native ACL instead of replacing it with a graft has long been a goal of surgeons, and before the BEAR Implant, multiple approaches were studied and failed. The BEAR Implant, which is the first medical technology to show that it enables a patient’s own torn ACL to heal, represents the first substantial advancement in the treatment of ACL tears in decades and has the potential to change the standard of care.” – Martha Shadan, President and CEO, Miach Orthopaedics
Miach Orthopaedics gained FDA De Novo approval for the bioengineered Bridge-Enhanced® ACL Repair (BEAR®) Implant for the treatment of anterior cruciate ligament (ACL) tears. Limited market release is slated for early 2021.
BEAR is the first medical technology to clinically demonstrate that it enables healing/restoration of a torn ACL. This...
Miach Orthopaedics gained FDA De Novo approval for the bioengineered Bridge-Enhanced® ACL Repair (BEAR®) Implant for the treatment of anterior cruciate ligament (ACL) tears. Limited market release is slated for early 2021.
BEAR is the first medical technology to clinically demonstrate that it enables healing/restoration of a torn ACL. This approach presents an alternative to reconstruction that replaces the ACL with a graft, and is the first new treatment for ACL tears in more than 30 years.
BEAR is designed to restore natural anatomy and function of the knee, and does not require a second surgical procedure to remove a healthy tendon from another part of the leg or use of a deceased donor’s tendon that requires special storage and handling requirements.
The resorbable implant is made from bovine collagen and secured via suture to bridge the gap between the torn ends of a patient’s ACL. The patient’s own blood is injected into the implant to form a device-protected clot that enables the body’s healing process. Within about eight weeks the implant is absorbed and replaced by the body’s own tissue.Pre-clinical studies suggest that the BEAR Implant may reduce the rate of osteoarthritis, estimated to occur in 78% of people with ACL tears, but this has not yet been confirmed in human trials.
“Preserving a patient’s native ACL instead of replacing it with a graft has long been a goal of surgeons, and before the BEAR Implant, multiple approaches were studied and failed. The BEAR Implant, which is the first medical technology to show that it enables a patient’s own torn ACL to heal, represents the first substantial advancement in the treatment of ACL tears in decades and has the potential to change the standard of care.” – Martha Shadan, President and CEO, Miach Orthopaedics

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







