
 Copy to clipboard
Copy to clipboard 
The Cleveland Clinic completed enrollment in the BEAR MOON study comparing Miach Orthopaedics’ BEAR Implant to autograft bone-patellar tendon-bone (BPTB) ACL reconstruction.
BEAR MOON is a randomized, controlled clinical trial led by the Cleveland Clinic and conducted at six clinical centers of excellence. The study enrolled 150 patients between 18 and 55 years of age with complete ACL tears who were randomized during surgery to treatment with either the BEAR Implant or BPTB ACLR.
“Our significant body of clinical evidence for the BEAR Implant, extending out 10 years, has shown consistently positive results,” said Patrick McBrayer, president and CEO, Miach Orthopaedics. “By comparing the BEAR Implant to standard of care ACLR, the BEAR MOON study provides the opportunity to build on these results in a multi-center study at six of the leading orthopaedic institutions in the U.S. We would like to thank the BEAR MOON investigators and clinical study site teams for their commitment to this study since 2018, and we look forward to the results and the impact they may have on the field of sports medicine.”
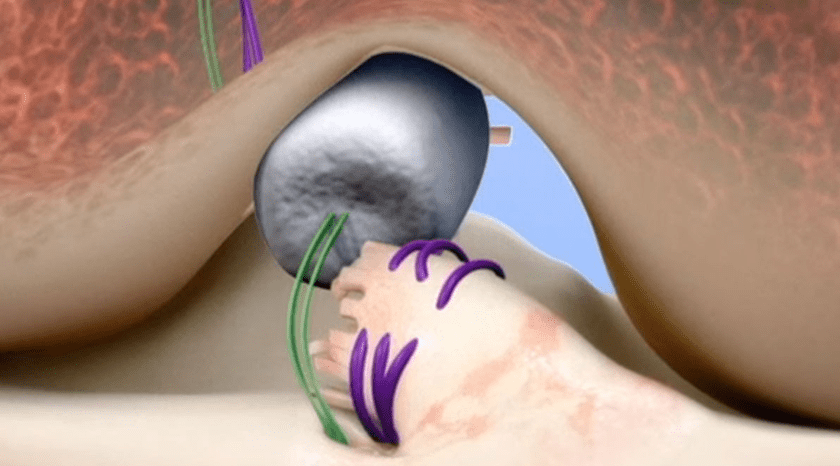
The BEAR (Bridge-Enhanced ACL Restoration) Implant is a proprietary collagen-based implant used to facilitate healing of the torn ACL.
The implant was first granted De Novo Approval from FDA in 2020.
Source: Miach Orthopaedics, Inc.
The Cleveland Clinic completed enrollment in the BEAR MOON study comparing Miach Orthopaedics’ BEAR Implant to autograft bone-patellar tendon-bone (BPTB) ACL reconstruction.
BEAR MOON is a randomized, controlled clinical trial led by the Cleveland Clinic and conducted at six clinical centers of excellence. The study enrolled 150 patients...
The Cleveland Clinic completed enrollment in the BEAR MOON study comparing Miach Orthopaedics’ BEAR Implant to autograft bone-patellar tendon-bone (BPTB) ACL reconstruction.
BEAR MOON is a randomized, controlled clinical trial led by the Cleveland Clinic and conducted at six clinical centers of excellence. The study enrolled 150 patients between 18 and 55 years of age with complete ACL tears who were randomized during surgery to treatment with either the BEAR Implant or BPTB ACLR.
“Our significant body of clinical evidence for the BEAR Implant, extending out 10 years, has shown consistently positive results,” said Patrick McBrayer, president and CEO, Miach Orthopaedics. “By comparing the BEAR Implant to standard of care ACLR, the BEAR MOON study provides the opportunity to build on these results in a multi-center study at six of the leading orthopaedic institutions in the U.S. We would like to thank the BEAR MOON investigators and clinical study site teams for their commitment to this study since 2018, and we look forward to the results and the impact they may have on the field of sports medicine.”
The BEAR (Bridge-Enhanced ACL Restoration) Implant is a proprietary collagen-based implant used to facilitate healing of the torn ACL.
The implant was first granted De Novo Approval from FDA in 2020.
Source: Miach Orthopaedics, Inc.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







