
 Copy to clipboard
Copy to clipboard 
Two-year study results indicate that use of the Bridge-Enhanced® ACL Repair (BEAR®) procedure from Miach Orthopaedics resulted in similar clinical, functional and patient-reported outcomes as autograft anterior cruciate ligament (ACL) reconstruction. (The supporting human study data can be found here, “Bridge-Enhanced Anterior Cruciate Ligament Repair: Two-Year Results of a First-in-Human Study.”)
BEAR is a bio-engineered sponge used as a bridging scaffold to stimulate healing of a torn ACL. Unlike traditional ACL reconstruction, the procedure does not require grafts from healthy parts of the leg.
The BEAR I study also showed that BEAR recipients showed no infection or severe inflammatory response, arthrofibrosis or a reaction that required scaffold removal. Manual and instrumented measures suggest that the stability of the knee after both BEAR and autograft ACL reconstruction may be comparable.
BEAR I was a non-randomized, two-arm study comparing 10 BEAR patients to 10 patients treated with autograft ACL reconstruction. Enrollment of 100 patients is complete for BEAR II, a randomized, blinded trial, and Miach is planning a third study to investigate the effects of patient age on outcomes.
Source: Miach Orthopaedics, Inc.
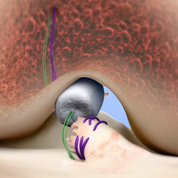
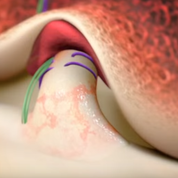
Images courtesy of Miach Orthopaedics
Two-year study results indicate that use of the Bridge-Enhanced® ACL Repair (BEAR®) procedure from Miach Orthopaedics resulted in similar clinical, functional and patient-reported outcomes as autograft anterior cruciate ligament (ACL) reconstruction. (The supporting human study data can be found here, "Bridge-Enhanced Anterior Cruciate Ligament...
Two-year study results indicate that use of the Bridge-Enhanced® ACL Repair (BEAR®) procedure from Miach Orthopaedics resulted in similar clinical, functional and patient-reported outcomes as autograft anterior cruciate ligament (ACL) reconstruction. (The supporting human study data can be found here, “Bridge-Enhanced Anterior Cruciate Ligament Repair: Two-Year Results of a First-in-Human Study.”)
BEAR is a bio-engineered sponge used as a bridging scaffold to stimulate healing of a torn ACL. Unlike traditional ACL reconstruction, the procedure does not require grafts from healthy parts of the leg.
The BEAR I study also showed that BEAR recipients showed no infection or severe inflammatory response, arthrofibrosis or a reaction that required scaffold removal. Manual and instrumented measures suggest that the stability of the knee after both BEAR and autograft ACL reconstruction may be comparable.
BEAR I was a non-randomized, two-arm study comparing 10 BEAR patients to 10 patients treated with autograft ACL reconstruction. Enrollment of 100 patients is complete for BEAR II, a randomized, blinded trial, and Miach is planning a third study to investigate the effects of patient age on outcomes.
Source: Miach Orthopaedics, Inc.


Images courtesy of Miach Orthopaedics

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







