Copy to clipboard
Copy to clipboard 
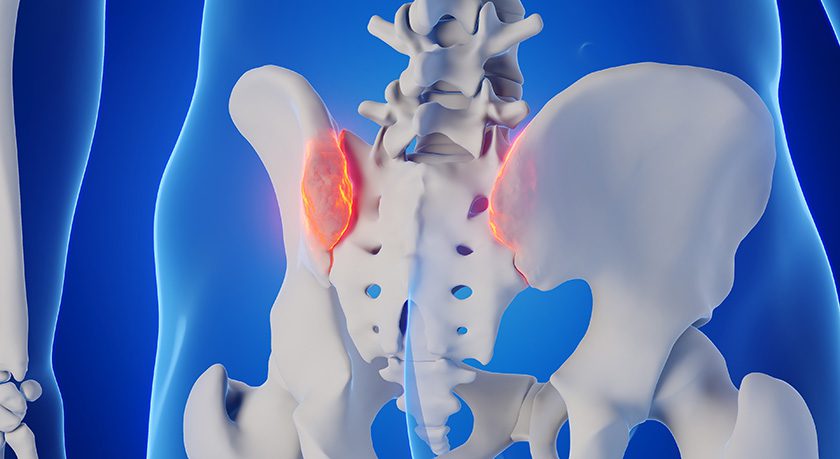
Medtronic gained FDA 510(k) clearance to market Kyphon™ HV-R Bone Cement for fixation of fractures of the sacral vertebral body using sacroplasty. This expands on earlier indications for treatment of vertebral compression fractures caused by osteoporosis, cancer or benign lesions.
In 1Q17, the company received FDA 510(k) clearance of Kyphon™ Xpede Bone Cement for fixation of pathological fractures of the sacral vertebral body, using sacroplasty. Kyphon Xpede is a quick-to-dough bone cement that, when paired with the Kyphon Cement Delivery System, may minimize radiation exposure for clinicians by standing up to four feet away from the radiation source during injection, which has been measured to reduce hand radiation exposure by 70%.
Sources: Medtronic plc; ORTHOWORLD Inc.
Medtronic gained FDA 510(k) clearance to market Kyphon™ HV-R Bone Cement for fixation of fractures of the sacral vertebral body using sacroplasty. This expands on earlier indications for treatment of vertebral compression fractures caused by osteoporosis, cancer or benign lesions.
In 1Q17, the company received FDA 510(k) clearance of...
Medtronic gained FDA 510(k) clearance to market Kyphon™ HV-R Bone Cement for fixation of fractures of the sacral vertebral body using sacroplasty. This expands on earlier indications for treatment of vertebral compression fractures caused by osteoporosis, cancer or benign lesions.
In 1Q17, the company received FDA 510(k) clearance of Kyphon™ Xpede Bone Cement for fixation of pathological fractures of the sacral vertebral body, using sacroplasty. Kyphon Xpede is a quick-to-dough bone cement that, when paired with the Kyphon Cement Delivery System, may minimize radiation exposure for clinicians by standing up to four feet away from the radiation source during injection, which has been measured to reduce hand radiation exposure by 70%.
Sources: Medtronic plc; ORTHOWORLD Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.