
 Copy to clipboard
Copy to clipboard 
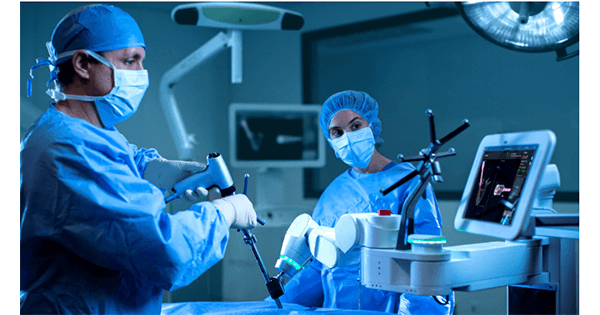
Medtronic was granted FDA 510(k) clearance to market the use of navigated interbody and Midas Rex™ high speed drills with the Mazor™ Robotic Guidance System.
The Mazor platform allows surgeons to quickly visualize anatomy and spinal structures in relation to one another in 3D. The interface delivers fast, seamless access to plan and simulate cages and screws to help enhance efficiency in the surgical workflow. Midas Rex High Speed Drill Systems are now fully integrated throughout the Mazor procedure, enabling trajectory precision starting with pilot hole creation and offering attachments and dissecting tools designed for accurate drilling with speeds up to 75,000 rpm.
Further, surgeons can now use navigated interbody features on the Mazor system to visualize disc prep and interbody placement during a robotic procedure.
Linnea Burman, Vice President & General Manager, Enabling Technologies: Cranial & Spinal, said, “With these recent clearances, we have advanced the Mazor platform by increasing its utility, improving its precision and adding capabilities as we drive toward our goal of delivering value-driven solutions for unmet needs.”
Medtronic was granted FDA 510(k) clearance to market the use of navigated interbody and Midas Rex™ high speed drills with the Mazor™ Robotic Guidance System.
The Mazor platform allows surgeons to quickly visualize anatomy and spinal structures in relation to one another in 3D. The interface delivers fast, seamless access to plan and...
Medtronic was granted FDA 510(k) clearance to market the use of navigated interbody and Midas Rex™ high speed drills with the Mazor™ Robotic Guidance System.
The Mazor platform allows surgeons to quickly visualize anatomy and spinal structures in relation to one another in 3D. The interface delivers fast, seamless access to plan and simulate cages and screws to help enhance efficiency in the surgical workflow. Midas Rex High Speed Drill Systems are now fully integrated throughout the Mazor procedure, enabling trajectory precision starting with pilot hole creation and offering attachments and dissecting tools designed for accurate drilling with speeds up to 75,000 rpm.
Further, surgeons can now use navigated interbody features on the Mazor system to visualize disc prep and interbody placement during a robotic procedure.
Linnea Burman, Vice President & General Manager, Enabling Technologies: Cranial & Spinal, said, “With these recent clearances, we have advanced the Mazor platform by increasing its utility, improving its precision and adding capabilities as we drive toward our goal of delivering value-driven solutions for unmet needs.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







