Copy to clipboard
Copy to clipboard 
Following CE Mark approval earlier this month, Medovex received its first 3 commercial orders for the DenerveX™ system from distributors in Germany, Italy and the U.K.
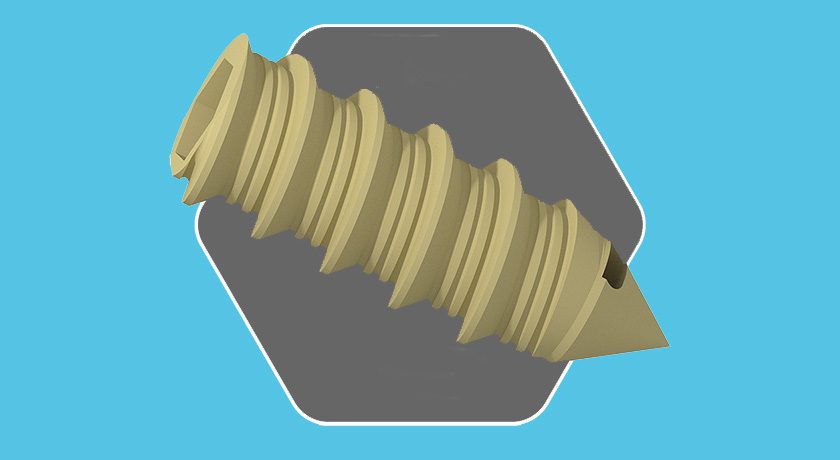
DenerveX comprises a single-use device and power generator. Intended for the treatment of Facet Joint Syndrome, the system denervates and removes capsular tissue from the facet joint in a single procedure that combines deburring action and RF ablation. The burr removes targeted synovial membranes and joint surfaces, while heat ablation destroys tissue and removes any residual nervous and synovial membrane overlying the joint, removing the end point sensory tissue.
Sources: Medovex Corporation; ORTHOWORLD Inc.
Following CE Mark approval earlier this month, Medovex received its first 3 commercial orders for the DenerveX™ system from distributors in Germany, Italy and the U.K.
DenerveX comprises a single-use device and power generator. Intended for the treatment of Facet Joint Syndrome, the system denervates and removes capsular tissue from the facet...
Following CE Mark approval earlier this month, Medovex received its first 3 commercial orders for the DenerveX™ system from distributors in Germany, Italy and the U.K.
DenerveX comprises a single-use device and power generator. Intended for the treatment of Facet Joint Syndrome, the system denervates and removes capsular tissue from the facet joint in a single procedure that combines deburring action and RF ablation. The burr removes targeted synovial membranes and joint surfaces, while heat ablation destroys tissue and removes any residual nervous and synovial membrane overlying the joint, removing the end point sensory tissue.
Sources: Medovex Corporation; ORTHOWORLD Inc.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.