
 Copy to clipboard
Copy to clipboard 
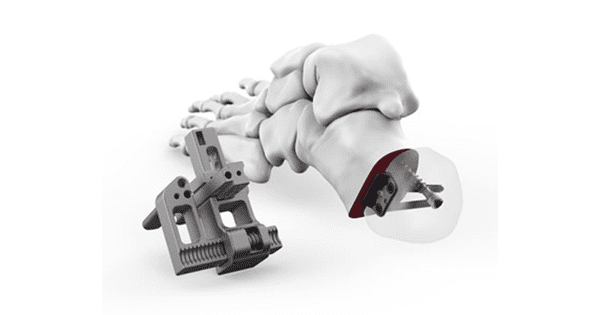
Medartis received FDA 510(k) clearance to market its CalcShift System for displacement calcaneal osteotomies. The system is one of several new lower extremity products scheduled for launch in the U.S. in 2023.
Indicated for osteotomies, non-unions, malunions, revisions, fusions and reconstruction of the calcaneus requiring a medial or lateral displacement osteotomy, the new CalcShift system features a streamlined instrumentation platform allowing surgeons to perform minimally invasive or traditional open medial or lateral displacement osteotomies with consistently reproducible results.
CalcShift, which features a low-profile pocket plate with locking and non-locking screws as well headless cannulated screw options, is the latest offering that Medartis has developed in partnership with its U.S. surgeon design team. Surgeons have the option of performing a calcaneal dislocation osteotomy minimally invasive or using a traditional open technique without additional assistance in the OR.
The CalcShift Displacement Calcaneal Osteotomy System is manufactured by Nextremity Solutions, which Medartis acquired in May.
“We’re excited about the recent 510(k) clearance of our new CalcShift system,” said Ryan Schlotterback, Chief Technology Officer at Medartis. “This system is another great example of our surgeon partners helping us create an instrumented system to assist them in making what can be perceived as a challenging procedure easy and repeatable while giving them confidence in achieving the desired correction. As we’ve done in the past, the team has created a well-instrumented system that provides our surgeons multiple fixation options.”
Source: Medartis
Medartis received FDA 510(k) clearance to market its CalcShift System for displacement calcaneal osteotomies. The system is one of several new lower extremity products scheduled for launch in the U.S. in 2023.
Indicated for osteotomies, non-unions, malunions, revisions, fusions and reconstruction of the calcaneus requiring a medial or lateral...
Medartis received FDA 510(k) clearance to market its CalcShift System for displacement calcaneal osteotomies. The system is one of several new lower extremity products scheduled for launch in the U.S. in 2023.
Indicated for osteotomies, non-unions, malunions, revisions, fusions and reconstruction of the calcaneus requiring a medial or lateral displacement osteotomy, the new CalcShift system features a streamlined instrumentation platform allowing surgeons to perform minimally invasive or traditional open medial or lateral displacement osteotomies with consistently reproducible results.
CalcShift, which features a low-profile pocket plate with locking and non-locking screws as well headless cannulated screw options, is the latest offering that Medartis has developed in partnership with its U.S. surgeon design team. Surgeons have the option of performing a calcaneal dislocation osteotomy minimally invasive or using a traditional open technique without additional assistance in the OR.
The CalcShift Displacement Calcaneal Osteotomy System is manufactured by Nextremity Solutions, which Medartis acquired in May.
“We’re excited about the recent 510(k) clearance of our new CalcShift system,” said Ryan Schlotterback, Chief Technology Officer at Medartis. “This system is another great example of our surgeon partners helping us create an instrumented system to assist them in making what can be perceived as a challenging procedure easy and repeatable while giving them confidence in achieving the desired correction. As we’ve done in the past, the team has created a well-instrumented system that provides our surgeons multiple fixation options.”
Source: Medartis

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







