
 Copy to clipboard
Copy to clipboard 
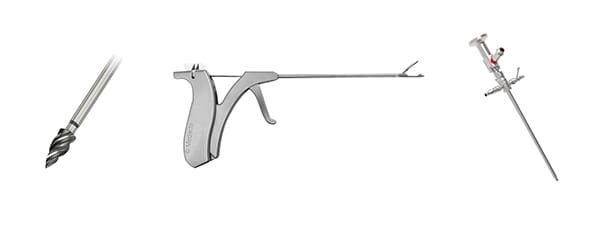
Medacta announced the first surgeries utilizing its MectaTap TI Anchor (above, left) and FastShuttle Suture Passer (center). The company’s Sports Medicine business has also obtained CE marking for various devices for hip, knee and shoulder arthroscopy.
The first worldwide MectaTap TI procedures were performed in the U.S. in several rotator cuff surgeries, alongside the FastShuttle for passing sutures.
CE Mark approval was gained for the following products:
- MectaFix Continuous Loop and Mectascrew B absorbable interference screw for ACL knee reconstructions
- PowerKnot High Strength Suture, a strong USP#2 suture with reliable knot tying features
- FastShuttle Suture Passer to facilitate passing and retrieving sutures in rotator cuff repairs
- FastShuttle Suture Passer cc for hip capsular closure
- MectaScope for hip and shoulder arthroscopy (above, right)
The company plans to add more products to the portfolio in coming months.
Medacta announced the first surgeries utilizing its MectaTap TI Anchor (above, left) and FastShuttle Suture Passer (center). The company's Sports Medicine business has also obtained CE marking for various devices for hip, knee and shoulder arthroscopy.
The first worldwide MectaTap TI procedures were performed in the U.S. in several rotator...
Medacta announced the first surgeries utilizing its MectaTap TI Anchor (above, left) and FastShuttle Suture Passer (center). The company’s Sports Medicine business has also obtained CE marking for various devices for hip, knee and shoulder arthroscopy.
The first worldwide MectaTap TI procedures were performed in the U.S. in several rotator cuff surgeries, alongside the FastShuttle for passing sutures.
CE Mark approval was gained for the following products:
- MectaFix Continuous Loop and Mectascrew B absorbable interference screw for ACL knee reconstructions
- PowerKnot High Strength Suture, a strong USP#2 suture with reliable knot tying features
- FastShuttle Suture Passer to facilitate passing and retrieving sutures in rotator cuff repairs
- FastShuttle Suture Passer cc for hip capsular closure
- MectaScope for hip and shoulder arthroscopy (above, right)
The company plans to add more products to the portfolio in coming months.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







