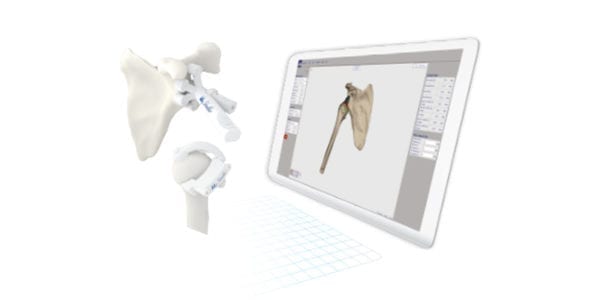






Medacta gained Pharmaceutical and Medical Device Agency approval for MyShoulder Placement Guides in Japan. Market launch will follow shortly; the company recently launched its shoulder implants in the region.
The patient-specific, 3D-printed devices comprise a humeral cutting guide, glenoidal reaming guide and a WebPlanner for pre-operative use.
Medacta’s MySolutions are personalized pre-operative planning and implant placement systems for use in hip, knee and spine procedures.
“The development of highly patient-personalized pre-operative planning and implant placement methodologies using our MySolutions technology has been one of our key innovations,” said Francesco Siccardi, CEO of Medacta International. “The introduction of the MyShoulder system is an important step to further expand our activities in the Japanese market.”
Source: Medacta International
Medacta gained Pharmaceutical and Medical Device Agency approval for MyShoulder Placement Guides in Japan. Market launch will follow shortly; the company recently launched its shoulder implants in the region.
The patient-specific, 3D-printed devices comprise a humeral cutting guide, glenoidal reaming guide and a WebPlanner for pre-operative...
Medacta gained Pharmaceutical and Medical Device Agency approval for MyShoulder Placement Guides in Japan. Market launch will follow shortly; the company recently launched its shoulder implants in the region.
The patient-specific, 3D-printed devices comprise a humeral cutting guide, glenoidal reaming guide and a WebPlanner for pre-operative use.
Medacta’s MySolutions are personalized pre-operative planning and implant placement systems for use in hip, knee and spine procedures.
“The development of highly patient-personalized pre-operative planning and implant placement methodologies using our MySolutions technology has been one of our key innovations,” said Francesco Siccardi, CEO of Medacta International. “The introduction of the MyShoulder system is an important step to further expand our activities in the Japanese market.”
Source: Medacta International


You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







