
 Copy to clipboard
Copy to clipboard 
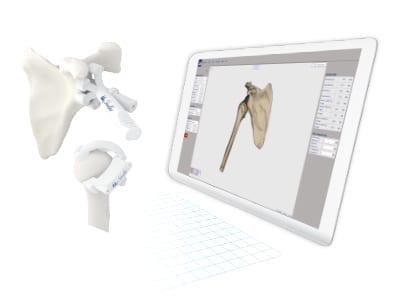
Medacta received FDA 510(k) clearance to market MyShoulder Placement Guides for use with the Medacta Shoulder and associated instrumentation, reportedly the first patient-specific offering in the U.S. to provide both humeral and glenoid guides in the surgical plan.
The company’s other personalized surgical approaches include MyKnee, MyHip and MySpine platforms, which have resulted in standard-of-care breakthroughs for joint replacement and spine surgery. MyShoulder Placement Guides will be added to the educational offering of Medacta’s Shoulder Learning Centers in the U.S., organized by the Medacta Orthopaedic Research and Education (M.O.R.E.) Institute.
Previously, Medacta launched the shoulder placement guides in Japan in 2019.
“Working with the MyShoulder Placement Guides enables me to play a key role in the surgical process from start to finish. Access to pre-operative planning and the support of a dedicated technician are key aspects of the platform and ensure the resulting guides are optimized for each procedure and patient,” said Matthew D. Saltzman, M.D., Associate Professor of Orthopaedic Surgery at the Northwestern Memorial Hospital in Chicago, Illinois.
Medacta received FDA 510(k) clearance to market MyShoulder Placement Guides for use with the Medacta Shoulder and associated instrumentation, reportedly the first patient-specific offering in the U.S. to provide both humeral and glenoid guides in the surgical plan.
The company's other personalized surgical approaches include MyKnee, MyHip and...
Medacta received FDA 510(k) clearance to market MyShoulder Placement Guides for use with the Medacta Shoulder and associated instrumentation, reportedly the first patient-specific offering in the U.S. to provide both humeral and glenoid guides in the surgical plan.
The company’s other personalized surgical approaches include MyKnee, MyHip and MySpine platforms, which have resulted in standard-of-care breakthroughs for joint replacement and spine surgery. MyShoulder Placement Guides will be added to the educational offering of Medacta’s Shoulder Learning Centers in the U.S., organized by the Medacta Orthopaedic Research and Education (M.O.R.E.) Institute.
Previously, Medacta launched the shoulder placement guides in Japan in 2019.
“Working with the MyShoulder Placement Guides enables me to play a key role in the surgical process from start to finish. Access to pre-operative planning and the support of a dedicated technician are key aspects of the platform and ensure the resulting guides are optimized for each procedure and patient,” said Matthew D. Saltzman, M.D., Associate Professor of Orthopaedic Surgery at the Northwestern Memorial Hospital in Chicago, Illinois.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







