
 Copy to clipboard
Copy to clipboard 
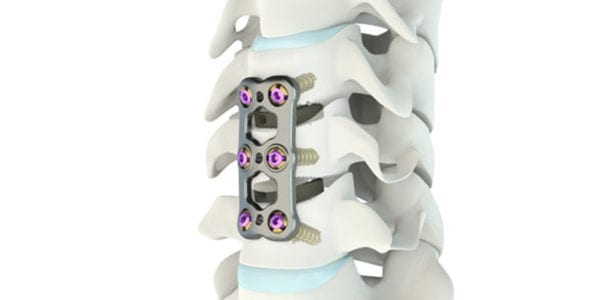
Medacta was granted FDA 510(k) clearance to market the Mecta-C Stand Alone system for anterior cervical discectomy and fusion.
Mecta-C’s screw system obviates the need for additional fixation. Modularity allows the surgeon to intraoperatively choose among four plating options – Flush, Trio, Hybrid and Quattro – to suit various patient needs and anatomies. Each option allows for either a locking or lag screw, and incorporates an easy-to-use locking mechanism.
Mecta-C Stand Alone are offered in TiPEEK, Medacta’s plasma-sprayed titanium coating to improve stability and increase migration resistance.
Medacta was granted FDA 510(k) clearance to market the Mecta-C Stand Alone system for anterior cervical discectomy and fusion.
Mecta-C's screw system obviates the need for additional fixation. Modularity allows the surgeon to intraoperatively choose among four plating options – Flush, Trio, Hybrid and Quattro – to suit various patient...
Medacta was granted FDA 510(k) clearance to market the Mecta-C Stand Alone system for anterior cervical discectomy and fusion.
Mecta-C’s screw system obviates the need for additional fixation. Modularity allows the surgeon to intraoperatively choose among four plating options – Flush, Trio, Hybrid and Quattro – to suit various patient needs and anatomies. Each option allows for either a locking or lag screw, and incorporates an easy-to-use locking mechanism.
Mecta-C Stand Alone are offered in TiPEEK, Medacta’s plasma-sprayed titanium coating to improve stability and increase migration resistance.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







