
 Copy to clipboard
Copy to clipboard 
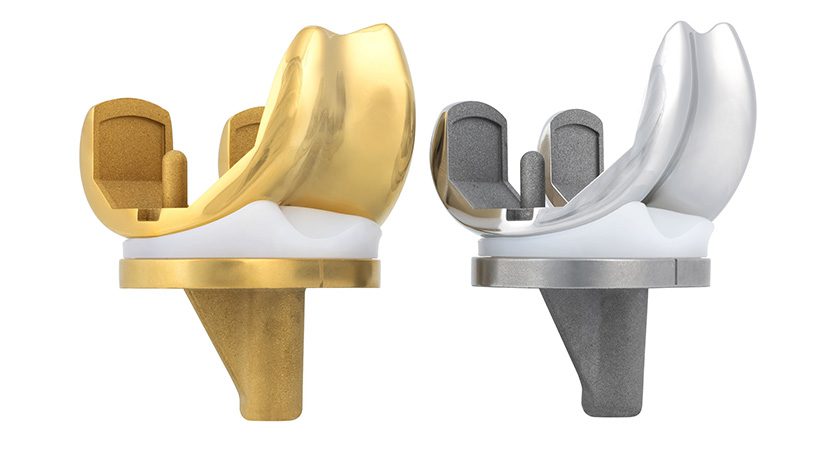
Maxx Orthopedics was granted FDA 510(k) clearance to market a Medial Congruent (MC) insert for the Freedom Total Knee. The insert will be available for clinical use in early 2025.
The new MC insert is designed to enhance total knee arthroplasty performance by incorporating features that better replicate natural knee function and motion. These include:
- Natural knee mechanics, with improved anterior sliding, lateral rollback, and external rotation.
- Medial stability, ensuring the medial compartment remains congruent throughout the full range of motion.
- Stability in extension, with a medial anterior lip to reduce anterior subluxation and enhance stability when the leg is in full extension.
“The Freedom Medial Congruent insert reflects Maxx Orthopedics’ commitment to continuous innovation,” said Ashesh Shah, Chief Executive Officer of Maxx Orthopedics, Inc. ”The Freedom Medial Congruent insert expands the Freedom Knee portfolio by providing a tibial insert designed to give patients knee movement, in cruciate-retaining procedures, that mimics the natural knee.”
Source: Maxx Orthopedics, Inc.
Maxx Orthopedics was granted FDA 510(k) clearance to market a Medial Congruent (MC) insert for the Freedom Total Knee. The insert will be available for clinical use in early 2025.
The new MC insert is designed to enhance total knee arthroplasty performance by incorporating features that better replicate natural knee function and motion. These...
Maxx Orthopedics was granted FDA 510(k) clearance to market a Medial Congruent (MC) insert for the Freedom Total Knee. The insert will be available for clinical use in early 2025.
The new MC insert is designed to enhance total knee arthroplasty performance by incorporating features that better replicate natural knee function and motion. These include:
- Natural knee mechanics, with improved anterior sliding, lateral rollback, and external rotation.
- Medial stability, ensuring the medial compartment remains congruent throughout the full range of motion.
- Stability in extension, with a medial anterior lip to reduce anterior subluxation and enhance stability when the leg is in full extension.
“The Freedom Medial Congruent insert reflects Maxx Orthopedics’ commitment to continuous innovation,” said Ashesh Shah, Chief Executive Officer of Maxx Orthopedics, Inc. ”The Freedom Medial Congruent insert expands the Freedom Knee portfolio by providing a tibial insert designed to give patients knee movement, in cruciate-retaining procedures, that mimics the natural knee.”
Source: Maxx Orthopedics, Inc.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







