
 Copy to clipboard
Copy to clipboard 
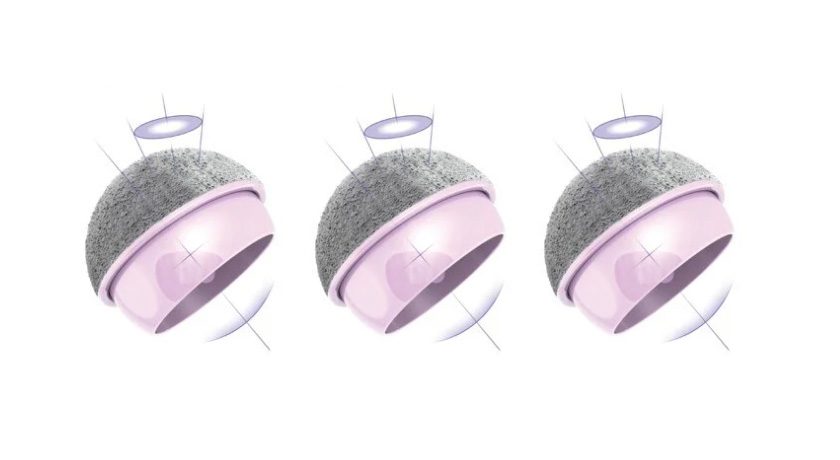
MatOrtho’s ReCerf Hip Resurfacing Arthroplasty has become the world’s first all-ceramic hip resurfacing system to receive regulatory approval, from the Therapeutic Goods Administration (TGA) in Australia.
ReCerf Hip Resurfacing launched in 2018; to date, over 1,200 patients have received the device and fewer have required further operation than usually anticipated with traditional hip replacement and preexisting hip resurfacing devices. In this year’s Annual Report, the Australian Orthopaedic Association National Joint Replacement Registry reported ReCerf to have a revision rate of just 0.3% (95% confidence intervals 0.0% to 2.1%) at three years.
TGA approval for ReCerf marks the first regulatory authorization of its kind globally. MatOrtho continues to work with the Australia Prosthesis List Advisory Committee to have ReCerf included on the next prosthesis list. They are also actively engaged in regulatory approvals in other key markets, to bring the benefits of Ceramic-on-Ceramic Hip Resurfacing with ReCerf to many more patients with the appropriate indications.
Mike Tuke, Founder of MatOrtho, said, “ReCerf’s all-ceramic ‘delta’ composition avoids concerns over metal implants, offering a safe and effective alternative to patients who can maintain more of their native hip and potentially avoid needing a conventional stemmed total hip replacement at all.”
Oriol Millet-Lopez, Interim CEO at MatOrtho, said, “ReCerf represents the culmination of years of innovation and dedication to improving patient outcomes. TGA approval underscores the system’s proven early performance and potential to redefine hip replacement globally.”
Source: MatOrtho
MatOrtho's ReCerf Hip Resurfacing Arthroplasty has become the world's first all-ceramic hip resurfacing system to receive regulatory approval, from the Therapeutic Goods Administration (TGA) in Australia.
ReCerf Hip Resurfacing launched in 2018; to date, over 1,200 patients have received the device and fewer have required further operation...
MatOrtho’s ReCerf Hip Resurfacing Arthroplasty has become the world’s first all-ceramic hip resurfacing system to receive regulatory approval, from the Therapeutic Goods Administration (TGA) in Australia.
ReCerf Hip Resurfacing launched in 2018; to date, over 1,200 patients have received the device and fewer have required further operation than usually anticipated with traditional hip replacement and preexisting hip resurfacing devices. In this year’s Annual Report, the Australian Orthopaedic Association National Joint Replacement Registry reported ReCerf to have a revision rate of just 0.3% (95% confidence intervals 0.0% to 2.1%) at three years.
TGA approval for ReCerf marks the first regulatory authorization of its kind globally. MatOrtho continues to work with the Australia Prosthesis List Advisory Committee to have ReCerf included on the next prosthesis list. They are also actively engaged in regulatory approvals in other key markets, to bring the benefits of Ceramic-on-Ceramic Hip Resurfacing with ReCerf to many more patients with the appropriate indications.
Mike Tuke, Founder of MatOrtho, said, “ReCerf’s all-ceramic ‘delta’ composition avoids concerns over metal implants, offering a safe and effective alternative to patients who can maintain more of their native hip and potentially avoid needing a conventional stemmed total hip replacement at all.”
Oriol Millet-Lopez, Interim CEO at MatOrtho, said, “ReCerf represents the culmination of years of innovation and dedication to improving patient outcomes. TGA approval underscores the system’s proven early performance and potential to redefine hip replacement globally.”
Source: MatOrtho

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







