
 Copy to clipboard
Copy to clipboard 
Loci Orthopaedics closed an oversubscribed €12.8 million (~US $14 million) Series A financing. The round was led by new investors Seroba, Johnson & Johnson Innovation, JJDC, Inc., and the European Innovation Council Fund.
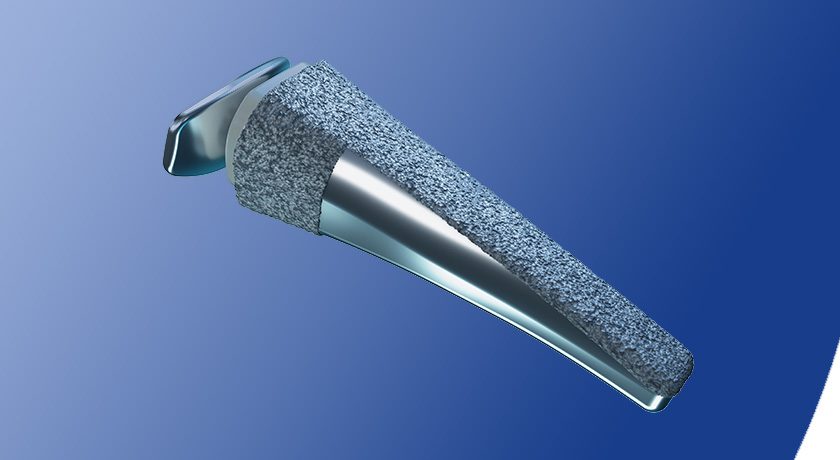
Loci Orthopaedics develops technologies that target major unmet clinical needs in orthopedic extremities. The company’s primary device, its patented InDx Implant System, is designed to treat thumb base joint arthritis. It replicates the functional biomechanics of the thumb base joint, with the aim of restoring natural motion. InDx’s novel design could offer an alternative to current thumb implants which may be prone to both dislocation and movement post-implantation.
The Series A financing will enable the company to augment the initial clinical investigation, which indicates positive preliminary results, and develop additional clinical data to support regulatory approval applications and future commercialization in different geographies.
In 2023, Loci Orthopaedics successfully completed enrollment in a 15-patient clinical study for InDx. The Thumb Hemi-Arthroplasty with Natural Kinematics study is designed to evaluate the surgical implantation of the device and improvements in pain, grip and quality of life for those affected by thumb base joint arthritis. Results from the clinical study are expected to be published later this year.
Dr Brendan Boland, Co-Founder and Executive Chairperson, commented: “Thumb base joint arthritis is a painful and disabling condition with a significant unmet clinical need for an effective, evidence-based, surgical solution. With a growing patient population, our InDx Implant System has the potential to provide surgeons and patients with a less invasive and more effective treatment for this condition. This funding will enable us to expand our clinical programs, submit regulatory approval applications in the US and EU and accelerate our efforts towards future commercialization.”
Barry Russell, CEO, added: “The company is excited to work with three very experienced and well-respected investment groups to help the company bring a promising new solution to market to help the many currently underserved patients and their surgeons with a joint sparing treatment option.”
Source: Loci Orthopaedics
Loci Orthopaedics closed an oversubscribed €12.8 million (~US $14 million) Series A financing. The round was led by new investors Seroba, Johnson & Johnson Innovation, JJDC, Inc., and the European Innovation Council Fund.
Loci Orthopaedics develops technologies that target major unmet clinical needs in orthopedic extremities. The company’s...
Loci Orthopaedics closed an oversubscribed €12.8 million (~US $14 million) Series A financing. The round was led by new investors Seroba, Johnson & Johnson Innovation, JJDC, Inc., and the European Innovation Council Fund.
Loci Orthopaedics develops technologies that target major unmet clinical needs in orthopedic extremities. The company’s primary device, its patented InDx Implant System, is designed to treat thumb base joint arthritis. It replicates the functional biomechanics of the thumb base joint, with the aim of restoring natural motion. InDx’s novel design could offer an alternative to current thumb implants which may be prone to both dislocation and movement post-implantation.
The Series A financing will enable the company to augment the initial clinical investigation, which indicates positive preliminary results, and develop additional clinical data to support regulatory approval applications and future commercialization in different geographies.
In 2023, Loci Orthopaedics successfully completed enrollment in a 15-patient clinical study for InDx. The Thumb Hemi-Arthroplasty with Natural Kinematics study is designed to evaluate the surgical implantation of the device and improvements in pain, grip and quality of life for those affected by thumb base joint arthritis. Results from the clinical study are expected to be published later this year.
Dr Brendan Boland, Co-Founder and Executive Chairperson, commented: “Thumb base joint arthritis is a painful and disabling condition with a significant unmet clinical need for an effective, evidence-based, surgical solution. With a growing patient population, our InDx Implant System has the potential to provide surgeons and patients with a less invasive and more effective treatment for this condition. This funding will enable us to expand our clinical programs, submit regulatory approval applications in the US and EU and accelerate our efforts towards future commercialization.”
Barry Russell, CEO, added: “The company is excited to work with three very experienced and well-respected investment groups to help the company bring a promising new solution to market to help the many currently underserved patients and their surgeons with a joint sparing treatment option.”
Source: Loci Orthopaedics

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







