
 Copy to clipboard
Copy to clipboard 
Locate Bio was granted Breakthrough Device designation by FDA for Chondro3, currently in development as a biomimetic graft for osteochondral lesions.
This is the company’s second FDA Breakthrough Device designation granted in 2021. In January, the company was granted a Breakthrough Device designation for CognitOss, an investigational treatment for chronic osteomyelitis. CognitOss is a drug/device combination product for the treatment of chronic infections within bone.
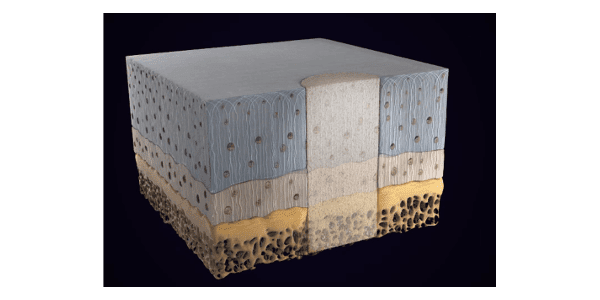
This current product, Chondro3, is a three-layered, proprietary collagen-based biodegradable matrix that can be delivered in a single procedure, in an outpatient setting and at an affordable price.
Chondro3 is designed to provide a scaffold for cellular and tissue in-growth and osteochondral defect repair at the site of lesion, supporting biomimetic repair of both cartilage and bone.
John von Benecke, CEO of Locate Bio, said, “There is an enduring unmet need for a cost-effective regenerative treatment [for osteochondral lesions], and we are proud that the FDA has recognized the potential of Chondro3 to address this serious public health issue. Having received two Breakthrough Device Designations this year, we are gaining real momentum towards achieving our ambition of building a world-leading orthobiologics business that addresses clear unmet needs of both surgeons and patients. We look forward to further progressing Chondro3 through the next stages of development to regulatory approval, and to discussing opportunities with potential partners who share our vision for this product.”
Locate Bio was granted Breakthrough Device designation by FDA for Chondro3, currently in development as a biomimetic graft for osteochondral lesions.
This is the company's second FDA Breakthrough Device designation granted in 2021. In January, the company was granted a Breakthrough Device designation for CognitOss, an investigational...
Locate Bio was granted Breakthrough Device designation by FDA for Chondro3, currently in development as a biomimetic graft for osteochondral lesions.
This is the company’s second FDA Breakthrough Device designation granted in 2021. In January, the company was granted a Breakthrough Device designation for CognitOss, an investigational treatment for chronic osteomyelitis. CognitOss is a drug/device combination product for the treatment of chronic infections within bone.
This current product, Chondro3, is a three-layered, proprietary collagen-based biodegradable matrix that can be delivered in a single procedure, in an outpatient setting and at an affordable price.
Chondro3 is designed to provide a scaffold for cellular and tissue in-growth and osteochondral defect repair at the site of lesion, supporting biomimetic repair of both cartilage and bone.
John von Benecke, CEO of Locate Bio, said, “There is an enduring unmet need for a cost-effective regenerative treatment [for osteochondral lesions], and we are proud that the FDA has recognized the potential of Chondro3 to address this serious public health issue. Having received two Breakthrough Device Designations this year, we are gaining real momentum towards achieving our ambition of building a world-leading orthobiologics business that addresses clear unmet needs of both surgeons and patients. We look forward to further progressing Chondro3 through the next stages of development to regulatory approval, and to discussing opportunities with potential partners who share our vision for this product.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







