
 Copy to clipboard
Copy to clipboard 
Wenzel Spine announced the completion of first cases and U.S. limited commercial launch of its novel panaSIa Expandable SI Fusion System. panaSIa is the first expandable sacroiliac (SI) fusion implant cleared by FDA. Full launch is slated for 4Q25.
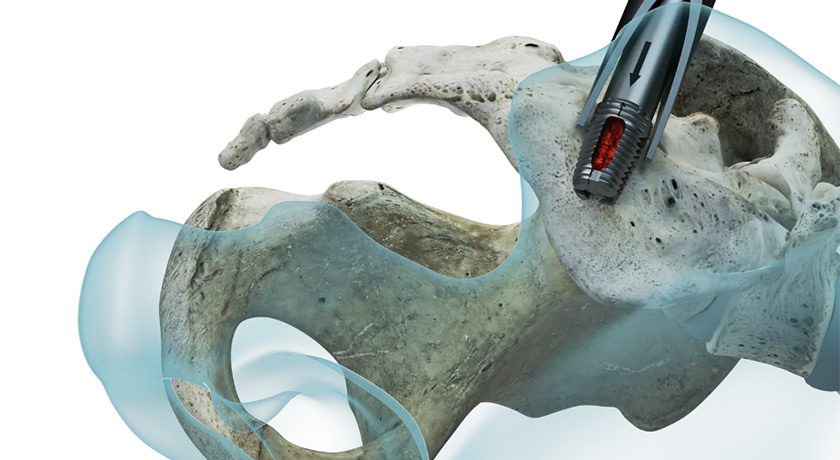
Designed to be implanted using a posterior, minimally invasive technique, panaSIa provides joint fixation through controlled expansion across the ilium and sacrum, offering immediate mechanical stability while preserving surrounding tissue. The implant’s integrated graft chamber allows for post-expansion bone grafting without disengaging instrumentation, facilitating optimal fusion conditions and minimizing procedural complexity.
“This launch is a defining milestone for Wenzel Spine,” said William Wilson, CEO of Wenzel Spine. “Our team has spent nearly two decades perfecting expandable device design, and panaSIa brings that legacy to a new clinical segment. We are proud to offer providers a differentiated, minimally invasive solution that transforms SI joint fusion with precision, control, and speed.”
Source: Wenzel Spine, Inc.
Wenzel Spine announced the completion of first cases and U.S. limited commercial launch of its novel panaSIa Expandable SI Fusion System. panaSIa is the first expandable sacroiliac (SI) fusion implant cleared by FDA. Full launch is slated for 4Q25.
Designed to be implanted using a posterior, minimally invasive technique, panaSIa provides...
Wenzel Spine announced the completion of first cases and U.S. limited commercial launch of its novel panaSIa Expandable SI Fusion System. panaSIa is the first expandable sacroiliac (SI) fusion implant cleared by FDA. Full launch is slated for 4Q25.
Designed to be implanted using a posterior, minimally invasive technique, panaSIa provides joint fixation through controlled expansion across the ilium and sacrum, offering immediate mechanical stability while preserving surrounding tissue. The implant’s integrated graft chamber allows for post-expansion bone grafting without disengaging instrumentation, facilitating optimal fusion conditions and minimizing procedural complexity.
“This launch is a defining milestone for Wenzel Spine,” said William Wilson, CEO of Wenzel Spine. “Our team has spent nearly two decades perfecting expandable device design, and panaSIa brings that legacy to a new clinical segment. We are proud to offer providers a differentiated, minimally invasive solution that transforms SI joint fusion with precision, control, and speed.”
Source: Wenzel Spine, Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.