Copy to clipboard
Copy to clipboard 
FDA approved the Investigational Device Exemption to start a clinical trial of LimaCorporate’s SMR Stemless Reverse Shoulder.
In 1Q21, Lima will commence a randomized, 7-site comparative clinical trial in the U.S. to compare SMR Stemless to the SMR Reverse Shoulder in total reverse shoulder arthroplasty. The trial will enroll 200 patients. SMR Stemless Reverse has been approved in Europe, Mexico and selected APAC markets including Australia, New Zealand and South Korea.
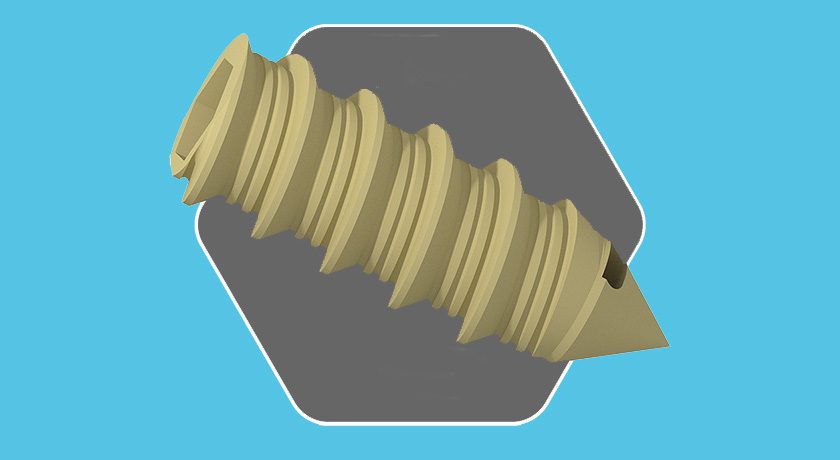
SMR Stemless Reverse Shoulder System is compatible with previously FDA-cleared SMR Reverse Shoulder components on the market since 2011. The Stemless Core features Trabecular Titanium (TT), LimaCorporate’s 3D-printed technology, and a reverse liner on the humeral side.
Luigi Ferrari, CEO of LimaCorporate, said, “The IDE study demonstrates LimaCorporate’s focus on improving patients’ lives through constant research and innovation, empowering and assisting surgeons to restore the emotion of motion in their patients.”
FDA approved the Investigational Device Exemption to start a clinical trial of LimaCorporate's SMR Stemless Reverse Shoulder.
In 1Q21, Lima will commence a randomized, 7-site comparative clinical trial in the U.S. to compare SMR Stemless to the SMR Reverse Shoulder in total reverse shoulder arthroplasty. The trial will enroll 200 patients....
FDA approved the Investigational Device Exemption to start a clinical trial of LimaCorporate’s SMR Stemless Reverse Shoulder.
In 1Q21, Lima will commence a randomized, 7-site comparative clinical trial in the U.S. to compare SMR Stemless to the SMR Reverse Shoulder in total reverse shoulder arthroplasty. The trial will enroll 200 patients. SMR Stemless Reverse has been approved in Europe, Mexico and selected APAC markets including Australia, New Zealand and South Korea.
SMR Stemless Reverse Shoulder System is compatible with previously FDA-cleared SMR Reverse Shoulder components on the market since 2011. The Stemless Core features Trabecular Titanium (TT), LimaCorporate’s 3D-printed technology, and a reverse liner on the humeral side.
Luigi Ferrari, CEO of LimaCorporate, said, “The IDE study demonstrates LimaCorporate’s focus on improving patients’ lives through constant research and innovation, empowering and assisting surgeons to restore the emotion of motion in their patients.”

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.