
 Copy to clipboard
Copy to clipboard 
Life Spine commenced a prospective study of the PROLIFT® Lateral Expandable System.
“Research and Clinical studies are incredibly important to our organization and to the spine community,” said Michael Butler, President and CEO of Life Spine. “The PROLIFT Lateral study is one of eight active studies being executed by Life Spine currently as a part of our dedication to researching the safety and efficacy of our products. Anecdotally, we have received excellent feedback regarding the PROLIFT Lateral System, but it is integral to our core goals to validate this feedback and ensure our products are advancing the standards of spine care.”
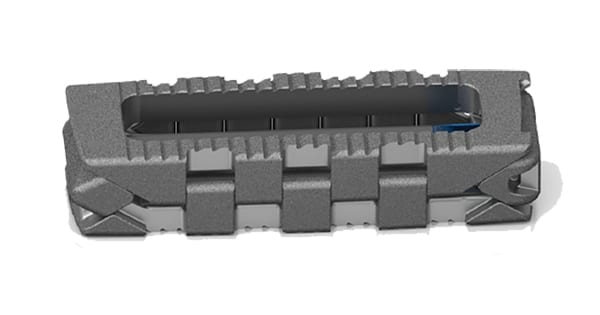
PROLIFT received initial FDA 510(k) clearance in 2019. The system features minimal insertion height and controlled, in situ expansion, and is complemented by the company’s full lateral portfolio: the CENTRIC® Retractor, Lateral Disc Prep, OSTEO-LINE® Graft Delivery device, neuromonitoring instruments, LONGBOW®Expandable Spacer and SENTRY® Lateral Plating.
Life Spine commenced a prospective study of the PROLIFT® Lateral Expandable System.
“Research and Clinical studies are incredibly important to our organization and to the spine community,” said Michael Butler, President and CEO of Life Spine. “The PROLIFT Lateral study is one of eight active studies being executed by Life Spine currently...
Life Spine commenced a prospective study of the PROLIFT® Lateral Expandable System.
“Research and Clinical studies are incredibly important to our organization and to the spine community,” said Michael Butler, President and CEO of Life Spine. “The PROLIFT Lateral study is one of eight active studies being executed by Life Spine currently as a part of our dedication to researching the safety and efficacy of our products. Anecdotally, we have received excellent feedback regarding the PROLIFT Lateral System, but it is integral to our core goals to validate this feedback and ensure our products are advancing the standards of spine care.”
PROLIFT received initial FDA 510(k) clearance in 2019. The system features minimal insertion height and controlled, in situ expansion, and is complemented by the company’s full lateral portfolio: the CENTRIC® Retractor, Lateral Disc Prep, OSTEO-LINE® Graft Delivery device, neuromonitoring instruments, LONGBOW®Expandable Spacer and SENTRY® Lateral Plating.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







