
 Copy to clipboard
Copy to clipboard 
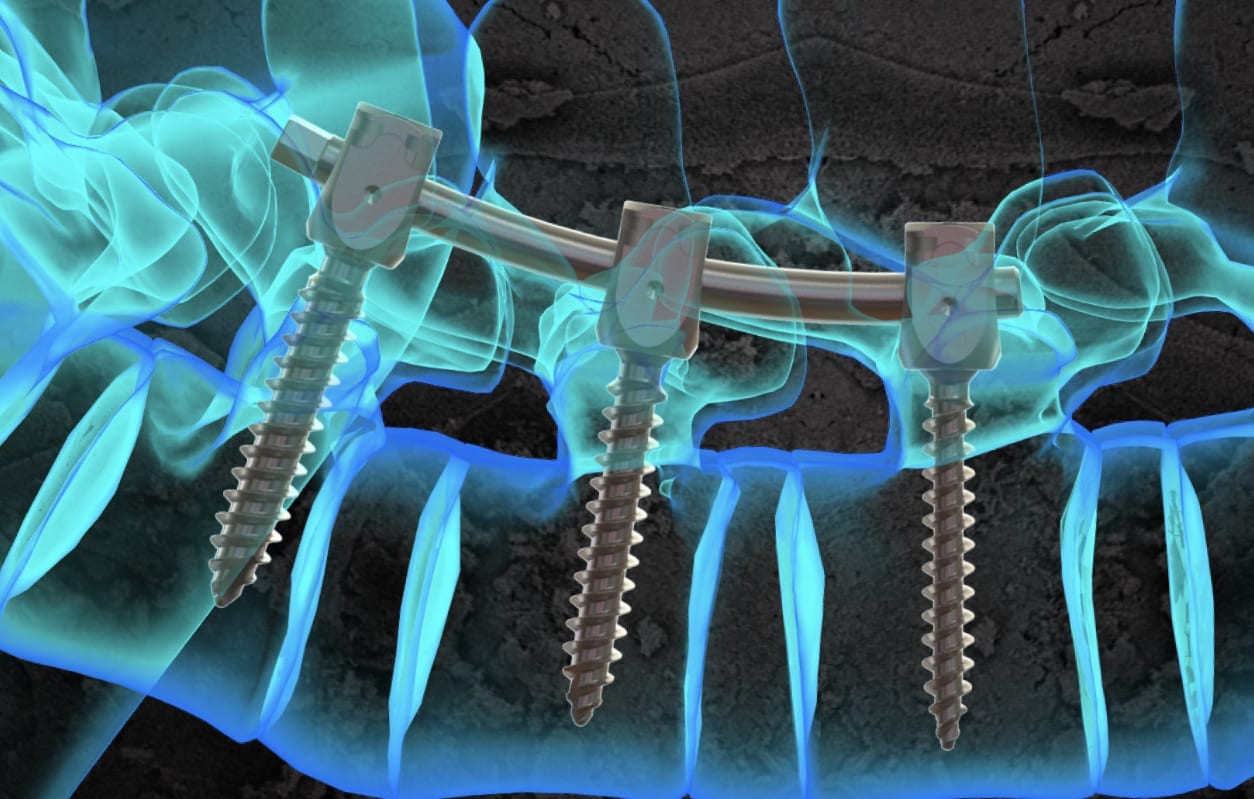
Life Spine was granted FDA 510(k) clearance to market implant and instrument additions to the ARx Spinal System. ARx received its original clearance in 2006; this is the system’s fourth clearance update.
ARx is Life Spine’s fourth 510(k) clearance in 2020, marking 92 clearances for the company overall. ARx is complemented by the PROLIFT® Expandable System and can be utilized with the SOLSTICE® Occiptio-Cervico-Thoracic System of hooks, prebent rods, transition components, cross connectors, etc.
“The additions to the ARx Spinal System will allow us to provide surgeons with the tools to accommodate a larger range of complex and deformity spinal procedures,” said Rich Mueller, Chief Operating Officer for Life Spine. “The ARx Spinal System is designed to advance the treatment for spinal deformity patients with a range of unique spinal implants and intuitive instrumentation which is consistently being enhanced by our research and development team to meet patient needs.”
Life Spine was granted FDA 510(k) clearance to market implant and instrument additions to the ARx Spinal System. ARx received its original clearance in 2006; this is the system's fourth clearance update.
ARx is Life Spine's fourth 510(k) clearance in 2020, marking 92 clearances for the company overall. ARx is complemented by the PROLIFT®...
Life Spine was granted FDA 510(k) clearance to market implant and instrument additions to the ARx Spinal System. ARx received its original clearance in 2006; this is the system’s fourth clearance update.
ARx is Life Spine’s fourth 510(k) clearance in 2020, marking 92 clearances for the company overall. ARx is complemented by the PROLIFT® Expandable System and can be utilized with the SOLSTICE® Occiptio-Cervico-Thoracic System of hooks, prebent rods, transition components, cross connectors, etc.
“The additions to the ARx Spinal System will allow us to provide surgeons with the tools to accommodate a larger range of complex and deformity spinal procedures,” said Rich Mueller, Chief Operating Officer for Life Spine. “The ARx Spinal System is designed to advance the treatment for spinal deformity patients with a range of unique spinal implants and intuitive instrumentation which is consistently being enhanced by our research and development team to meet patient needs.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







