
 Copy to clipboard
Copy to clipboard 
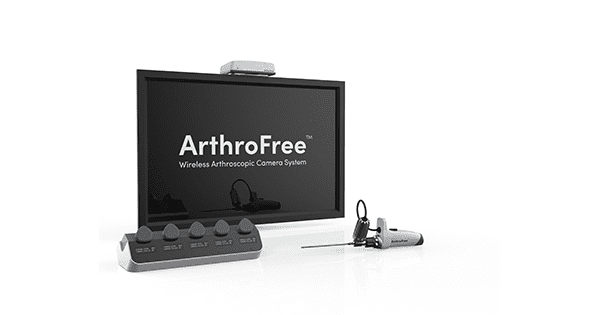
Lazurite (formerly Indago) has contracted with University Hospitals (UH) Ventures – the innovation and commercialization arm of University Hospitals Health System in Cleveland – to conduct a formative human factors study on its ArthroFree™ wireless surgical camera as part of a broader multisite study. Human factors, or usability studies, are intended to demonstrate the performance of a device within an environment that simulates its real environment of use and by people who are the potential users.
The ArthroFree system is expected to be the world’s first FDA-cleared fully wireless, minimally invasive camera system for the OR. It is expected to deliver OR efficiency gains, cost savings, improved safety and reduced sterilization requirements through eliminating cords and cables, and significant reduction of the risk of OR burns and fires with its proprietary low-heat, high-power light engine technology.
The modular ArthroFree system is designed to be fully drop-in compatible with current operating room technology. The coming market launch, following anticipated receipt of FDA market clearance in 2022, will initially focus on orthopedic applications, particularly in sports medicine.
Source: Lazurite
Lazurite (formerly Indago) has contracted with University Hospitals (UH) Ventures – the innovation and commercialization arm of University Hospitals Health System in Cleveland – to conduct a formative human factors study on its ArthroFree™ wireless surgical camera as part of a broader multisite study. Human factors, or usability studies, are...
Lazurite (formerly Indago) has contracted with University Hospitals (UH) Ventures – the innovation and commercialization arm of University Hospitals Health System in Cleveland – to conduct a formative human factors study on its ArthroFree™ wireless surgical camera as part of a broader multisite study. Human factors, or usability studies, are intended to demonstrate the performance of a device within an environment that simulates its real environment of use and by people who are the potential users.
The ArthroFree system is expected to be the world’s first FDA-cleared fully wireless, minimally invasive camera system for the OR. It is expected to deliver OR efficiency gains, cost savings, improved safety and reduced sterilization requirements through eliminating cords and cables, and significant reduction of the risk of OR burns and fires with its proprietary low-heat, high-power light engine technology.
The modular ArthroFree system is designed to be fully drop-in compatible with current operating room technology. The coming market launch, following anticipated receipt of FDA market clearance in 2022, will initially focus on orthopedic applications, particularly in sports medicine.
Source: Lazurite

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







