
 Copy to clipboard
Copy to clipboard 
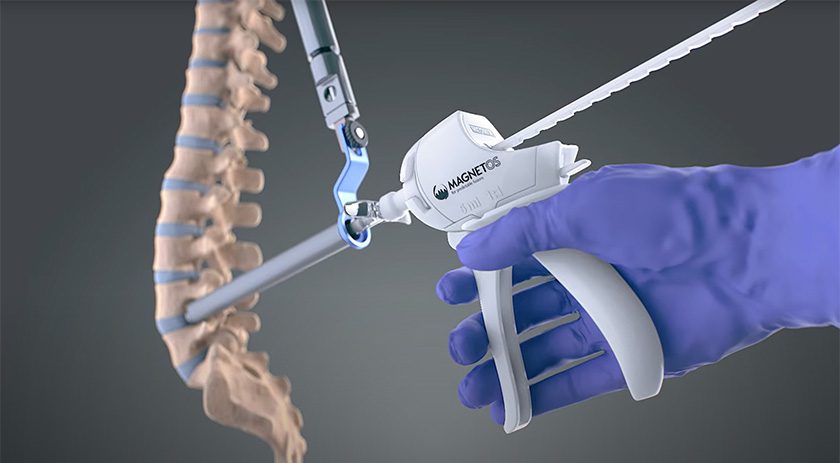
Kuros Biosciences announced completion of the first U.S. cases using the new MagnetOs MIS Delivery System, a sterile, prefilled, single-use delivery system engineered for Minimally Invasive Surgery (MIS) in spine procedures. This follows FDA 510(k) clearance of the MagnetOs MIS Delivery System in May.
MagnetOs MIS builds on the science of MagnetOs and its proprietary NeedleGrip submicron surface technology, which harnesses the immune system to stimulate bone growth. It is engineered for precise delivery, surgical efficiency and predictable fusion outcomes. Compared to delivering MagnetOs in a traditional, funnel-based system, MagnetOs MIS achieved graft placement three times faster, optimizing time in the operating room.
Additionally, and following the recent ANVISA approval of MagnetOs Granules, Kuros also announced that MagnetOs Putty now has been approved by the Brazilian regulatory authority, expanding the company’s entry into the South American spine and orthopedic market.
Chris Fair, CEO of Kuros Biosciences, commented: “With the first MagnetOs MIS case completed, we’re delivering what surgeons have been asking for – precision, speed, and proven outcomes when utilizing minimally invasive surgical techniques, which we know are on the rise. This unlocks access to the high-growth U.S. MIS spine segment. With the recent approval of MagnetOs Putty in Brazil, we’re accelerating access to our technology in key international markets where we see strong projected market growth.”
Source: Kuros Biosciences
Kuros Biosciences announced completion of the first U.S. cases using the new MagnetOs MIS Delivery System, a sterile, prefilled, single-use delivery system engineered for Minimally Invasive Surgery (MIS) in spine procedures. This follows FDA 510(k) clearance of the MagnetOs MIS Delivery System in May.
MagnetOs MIS builds on the science of...
Kuros Biosciences announced completion of the first U.S. cases using the new MagnetOs MIS Delivery System, a sterile, prefilled, single-use delivery system engineered for Minimally Invasive Surgery (MIS) in spine procedures. This follows FDA 510(k) clearance of the MagnetOs MIS Delivery System in May.
MagnetOs MIS builds on the science of MagnetOs and its proprietary NeedleGrip submicron surface technology, which harnesses the immune system to stimulate bone growth. It is engineered for precise delivery, surgical efficiency and predictable fusion outcomes. Compared to delivering MagnetOs in a traditional, funnel-based system, MagnetOs MIS achieved graft placement three times faster, optimizing time in the operating room.
Additionally, and following the recent ANVISA approval of MagnetOs Granules, Kuros also announced that MagnetOs Putty now has been approved by the Brazilian regulatory authority, expanding the company’s entry into the South American spine and orthopedic market.
Chris Fair, CEO of Kuros Biosciences, commented: “With the first MagnetOs MIS case completed, we’re delivering what surgeons have been asking for – precision, speed, and proven outcomes when utilizing minimally invasive surgical techniques, which we know are on the rise. This unlocks access to the high-growth U.S. MIS spine segment. With the recent approval of MagnetOs Putty in Brazil, we’re accelerating access to our technology in key international markets where we see strong projected market growth.”
Source: Kuros Biosciences

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







