
 Copy to clipboard
Copy to clipboard 
By revenue, the knee subsegment is the largest piece of the pie in joint replacement. For 2024, ORTHOWORLD estimates suggest that knee replacement sales will surpass $10.7 billion with growth of 6.6% vs. 2023. For 2025, we project knee sales to reach $11 billion. Here’s a look at the activity that occurred within this last quarter that will support growth in this well-performing segment for the coming year.
Corin Marks First Clinical Use of the Unity Knee MC Tibial Insert
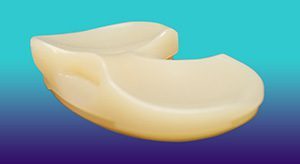 The Unity Knee MC (Medial Constrained) Tibial Insert is designed to recreate native knee kinematics by stabilizing the medial femoral condyle while allowing translation of the lateral femoral condyle. It can be used with or without the PCL. Combined with Corin’s ApolloKnee robotic-assisted solution for total knee replacement, surgeons can personalize the procedure based on a patient’s soft tissue envelope before making any bone cuts.
The Unity Knee MC (Medial Constrained) Tibial Insert is designed to recreate native knee kinematics by stabilizing the medial femoral condyle while allowing translation of the lateral femoral condyle. It can be used with or without the PCL. Combined with Corin’s ApolloKnee robotic-assisted solution for total knee replacement, surgeons can personalize the procedure based on a patient’s soft tissue envelope before making any bone cuts.
The insert is exclusively available in the United States through a limited market release.
Exactech Gains Clearance to Market the Truliant Porous 3D Tibial Implant
 The Truliant tray leverages additive manufacturing technology to build a porous structure that is designed to mimic the structure of cancellous bone. This design aims to facilitate both initial and biological fixation.
The Truliant tray leverages additive manufacturing technology to build a porous structure that is designed to mimic the structure of cancellous bone. This design aims to facilitate both initial and biological fixation.
The device features peripherally-placed tibial pegs, a dual v-channeled keel and optional cancellous bone screws designed to increase initial rotational stability and allow an increased bone/implant interface.
Maxx Orthopedics Gains FDA 510(k) for Medial Congruent Insert
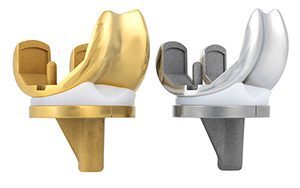 Maxx Ortho’s insert is designed to enhance total knee replacement performance through features that better replicate natural knee function and motion. These include improved anterior sliding, lateral rollback and external rotation; medial stability, ensuring the medial compartment remains congruent throughout the full range of motion; and stability in extension, with a medial anterior lip to reduce anterior subluxation and enhance stability when the leg is in full extension.
Maxx Ortho’s insert is designed to enhance total knee replacement performance through features that better replicate natural knee function and motion. These include improved anterior sliding, lateral rollback and external rotation; medial stability, ensuring the medial compartment remains congruent throughout the full range of motion; and stability in extension, with a medial anterior lip to reduce anterior subluxation and enhance stability when the leg is in full extension.
The insert will be available for clinical use in early 2025.
Smith+Nephew Launches LEGION Hinged Knee with OXINIUM
 The LEGION Hinged Knee (HK) landed in the U.S. LEGION HK is designed to provide a natural range of motion with medial pivot, lateral roll back and screw home. It allows surgeons to transition intraoperatively from a constrained revision knee implant to a CoCr-hinged assembly.
The LEGION Hinged Knee (HK) landed in the U.S. LEGION HK is designed to provide a natural range of motion with medial pivot, lateral roll back and screw home. It allows surgeons to transition intraoperatively from a constrained revision knee implant to a CoCr-hinged assembly.
The hinged knee is the latest expansion to the LEGION Total Knee System.
Total Joint Orthopedics Debuts Universal Cones
 The first revision total knee arthroplasties occurred using TJO’s Universal Cone System.
The first revision total knee arthroplasties occurred using TJO’s Universal Cone System.
Adding the cones to TJO’s Klassic Revision Knee allows the flexibility of a cruciate retaining primary through revision in only three trays of instruments. The anatomical sizing of the Universal Cone affords use in both the tibia and the femur, and supports a more bone-conserving approach while accommodating bone loss and patient anatomy. A guided ream-only technique ensures precise placement and a customized patient-fit implant.
Zimmer Biomet Gets Two Regulatory Nods
First, the company received 510(k) clearance to market the Persona SoluTion Porous Plasma Spray (PPS) Femur, a total knee implant component offering an alternative for patients with sensitivities to bone cement or metal. The device features a porous coating for cementless fixation and leverages a proprietary surface treatment designed to enhance wear performance. The components will be commercially available in the U.S. in 1Q25.
Further, they scored Premarket Approval Application Supplement approval for the Oxford Cementless Partial Knee.
 The system is designed to offer improved fixation, a better long-term implant survival rate and improved efficiency in the OR compared to the Oxford Cemented Partial Knee procedure. It is the only FDA-approved cementless partial knee in the U.S. The partial knee is accompanied by a lifetime limited warranty that covers the cost of Zimmer Biomet replacement implants.
The system is designed to offer improved fixation, a better long-term implant survival rate and improved efficiency in the OR compared to the Oxford Cemented Partial Knee procedure. It is the only FDA-approved cementless partial knee in the U.S. The partial knee is accompanied by a lifetime limited warranty that covers the cost of Zimmer Biomet replacement implants.
By revenue, the knee subsegment is the largest piece of the pie in joint replacement. For 2024, ORTHOWORLD estimates suggest that knee replacement sales will surpass $10.7 billion with growth of 6.6% vs. 2023. For 2025, we project knee sales to reach $11 billion. Here’s a look at the activity that occurred within this last quarter that will...
By revenue, the knee subsegment is the largest piece of the pie in joint replacement. For 2024, ORTHOWORLD estimates suggest that knee replacement sales will surpass $10.7 billion with growth of 6.6% vs. 2023. For 2025, we project knee sales to reach $11 billion. Here’s a look at the activity that occurred within this last quarter that will support growth in this well-performing segment for the coming year.
Corin Marks First Clinical Use of the Unity Knee MC Tibial Insert
 The Unity Knee MC (Medial Constrained) Tibial Insert is designed to recreate native knee kinematics by stabilizing the medial femoral condyle while allowing translation of the lateral femoral condyle. It can be used with or without the PCL. Combined with Corin’s ApolloKnee robotic-assisted solution for total knee replacement, surgeons can personalize the procedure based on a patient’s soft tissue envelope before making any bone cuts.
The Unity Knee MC (Medial Constrained) Tibial Insert is designed to recreate native knee kinematics by stabilizing the medial femoral condyle while allowing translation of the lateral femoral condyle. It can be used with or without the PCL. Combined with Corin’s ApolloKnee robotic-assisted solution for total knee replacement, surgeons can personalize the procedure based on a patient’s soft tissue envelope before making any bone cuts.
The insert is exclusively available in the United States through a limited market release.
Exactech Gains Clearance to Market the Truliant Porous 3D Tibial Implant
 The Truliant tray leverages additive manufacturing technology to build a porous structure that is designed to mimic the structure of cancellous bone. This design aims to facilitate both initial and biological fixation.
The Truliant tray leverages additive manufacturing technology to build a porous structure that is designed to mimic the structure of cancellous bone. This design aims to facilitate both initial and biological fixation.
The device features peripherally-placed tibial pegs, a dual v-channeled keel and optional cancellous bone screws designed to increase initial rotational stability and allow an increased bone/implant interface.
Maxx Orthopedics Gains FDA 510(k) for Medial Congruent Insert
 Maxx Ortho’s insert is designed to enhance total knee replacement performance through features that better replicate natural knee function and motion. These include improved anterior sliding, lateral rollback and external rotation; medial stability, ensuring the medial compartment remains congruent throughout the full range of motion; and stability in extension, with a medial anterior lip to reduce anterior subluxation and enhance stability when the leg is in full extension.
Maxx Ortho’s insert is designed to enhance total knee replacement performance through features that better replicate natural knee function and motion. These include improved anterior sliding, lateral rollback and external rotation; medial stability, ensuring the medial compartment remains congruent throughout the full range of motion; and stability in extension, with a medial anterior lip to reduce anterior subluxation and enhance stability when the leg is in full extension.
The insert will be available for clinical use in early 2025.
Smith+Nephew Launches LEGION Hinged Knee with OXINIUM
 The LEGION Hinged Knee (HK) landed in the U.S. LEGION HK is designed to provide a natural range of motion with medial pivot, lateral roll back and screw home. It allows surgeons to transition intraoperatively from a constrained revision knee implant to a CoCr-hinged assembly.
The LEGION Hinged Knee (HK) landed in the U.S. LEGION HK is designed to provide a natural range of motion with medial pivot, lateral roll back and screw home. It allows surgeons to transition intraoperatively from a constrained revision knee implant to a CoCr-hinged assembly.
The hinged knee is the latest expansion to the LEGION Total Knee System.
Total Joint Orthopedics Debuts Universal Cones
 The first revision total knee arthroplasties occurred using TJO’s Universal Cone System.
The first revision total knee arthroplasties occurred using TJO’s Universal Cone System.
Adding the cones to TJO’s Klassic Revision Knee allows the flexibility of a cruciate retaining primary through revision in only three trays of instruments. The anatomical sizing of the Universal Cone affords use in both the tibia and the femur, and supports a more bone-conserving approach while accommodating bone loss and patient anatomy. A guided ream-only technique ensures precise placement and a customized patient-fit implant.
Zimmer Biomet Gets Two Regulatory Nods
First, the company received 510(k) clearance to market the Persona SoluTion Porous Plasma Spray (PPS) Femur, a total knee implant component offering an alternative for patients with sensitivities to bone cement or metal. The device features a porous coating for cementless fixation and leverages a proprietary surface treatment designed to enhance wear performance. The components will be commercially available in the U.S. in 1Q25.
Further, they scored Premarket Approval Application Supplement approval for the Oxford Cementless Partial Knee.
 The system is designed to offer improved fixation, a better long-term implant survival rate and improved efficiency in the OR compared to the Oxford Cemented Partial Knee procedure. It is the only FDA-approved cementless partial knee in the U.S. The partial knee is accompanied by a lifetime limited warranty that covers the cost of Zimmer Biomet replacement implants.
The system is designed to offer improved fixation, a better long-term implant survival rate and improved efficiency in the OR compared to the Oxford Cemented Partial Knee procedure. It is the only FDA-approved cementless partial knee in the U.S. The partial knee is accompanied by a lifetime limited warranty that covers the cost of Zimmer Biomet replacement implants.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







