
 Copy to clipboard
Copy to clipboard 
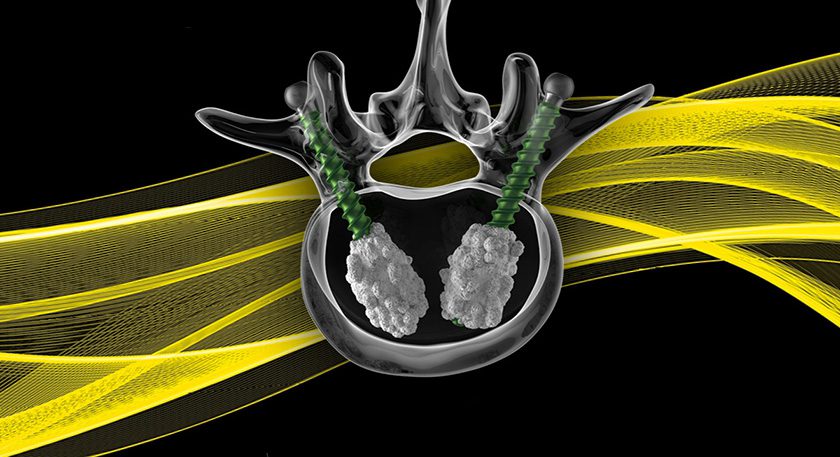
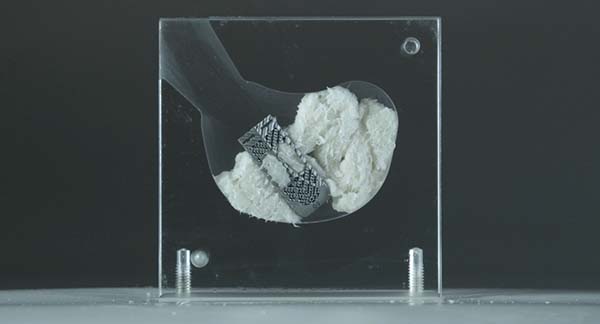
Kleiner Device Labs is showcasing the new KG™2 Surge™ flow-thru interbody system that recently received FDA 510(k) market clearance.
The new system maximizes total bone graft delivery volume, better distributes graft bilaterally into the intervertebral disc space, and streamlines the implant delivery, positioning and grafting process for TLIF and PLIF spinal fusion procedures. The implant comes preassembled to the inserter in a single-patient-use tray.
The company will next launch the product with a select group of surgeons and will be building inventory for its full commercial launch.
Source: Kleiner Device Labs
Kleiner Device Labs is showcasing the new KG™2 Surge™ flow-thru interbody system that recently received FDA 510(k) market clearance.
The new system maximizes total bone graft delivery volume, better distributes graft bilaterally into the intervertebral disc space, and streamlines the implant delivery, positioning and grafting process for TLIF...
Kleiner Device Labs is showcasing the new KG™2 Surge™ flow-thru interbody system that recently received FDA 510(k) market clearance.
The new system maximizes total bone graft delivery volume, better distributes graft bilaterally into the intervertebral disc space, and streamlines the implant delivery, positioning and grafting process for TLIF and PLIF spinal fusion procedures. The implant comes preassembled to the inserter in a single-patient-use tray.
The company will next launch the product with a select group of surgeons and will be building inventory for its full commercial launch.
Source: Kleiner Device Labs

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.