
 Copy to clipboard
Copy to clipboard 
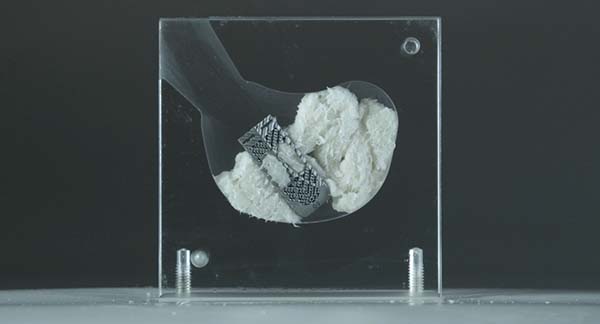
Kleiner Device Labs today announced that it was recently awarded a U.S. patent for its new KG®2 Surge® flow-thru interbody system. This adds to the company’s growing global portfolio of 35 issued patents and other intellectual property for spinal surgical technology developed by the company.
The new KG2 Surge flow-thru interbody system was developed with the objectives of maximizing bone graft delivery to the prepared intervertebral disc space, and streamlining implant placement, positioning, and integration in the graft matrix.
Founder and CEO Jeff Kleiner, MD, said, “We set out from the beginning to create answers for surgeons to maximize spinal fusion bone graft delivery and solve many of the most frustrating parts of spinal fusion procedures that negatively impact both the patient and the surgeon. Tabulating the results of our recently completed first 50 cases, we found that it took surgeons an average of only 8 minutes to trial, implant, graft and release the lumbar interbody device with the KG2 Surge system and that both the mean and median volume of graft delivered was 10ml.”
Source: PR Newswire
Kleiner Device Labs today announced that it was recently awarded a U.S. patent for its new KG®2 Surge® flow-thru interbody system. This adds to the company’s growing global portfolio of 35 issued patents and other intellectual property for spinal surgical technology developed by the company.
The new KG2 Surge flow-thru interbody system was...
Kleiner Device Labs today announced that it was recently awarded a U.S. patent for its new KG®2 Surge® flow-thru interbody system. This adds to the company’s growing global portfolio of 35 issued patents and other intellectual property for spinal surgical technology developed by the company.
The new KG2 Surge flow-thru interbody system was developed with the objectives of maximizing bone graft delivery to the prepared intervertebral disc space, and streamlining implant placement, positioning, and integration in the graft matrix.
Founder and CEO Jeff Kleiner, MD, said, “We set out from the beginning to create answers for surgeons to maximize spinal fusion bone graft delivery and solve many of the most frustrating parts of spinal fusion procedures that negatively impact both the patient and the surgeon. Tabulating the results of our recently completed first 50 cases, we found that it took surgeons an average of only 8 minutes to trial, implant, graft and release the lumbar interbody device with the KG2 Surge system and that both the mean and median volume of graft delivered was 10ml.”
Source: PR Newswire

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
ME
Mike Evers is a Senior Market Analyst and writer with over 15 years of experience in the medical industry, spanning cardiac rhythm management, ER coding and billing, and orthopedics. He joined ORTHOWORLD in 2018, where he provides market analysis and editorial coverage.







