
 Copy to clipboard
Copy to clipboard 
joimax received FDA 510(k) clearance to market the EndoLIF® Delta-Cage and EndoLIF® DoubleWedge-Cage. Delta-Cages and DoubleWedge-Cages are intended for intervertebral body fusion procedures for various diseases of the lumbar spine, such as degenerative disc disease.
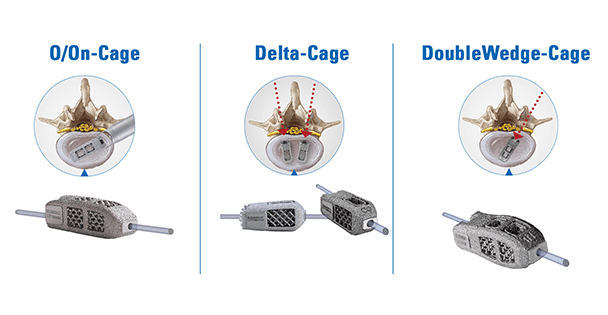
Now in its 20th anniversary year, joimax sets another milestone by providing a family of 3D printed titanium cages. The complete EndoLIF product portfolio consists of the Delta-Cage, the DoubleWedge-Cage, and the O/On-Cage. All three models are designed with special features:
- 3D printed titanium for excellent bone ingrowth, fillable with bone or bone substitute material, pre- or post-insertion
- Anatomical shape and lordotic correction options for OLIF, TLIF, and PLIF procedures
- “Over-the-wire” implantation for optimal targeted positioning in the intervertebral disc space
- Endoscopic control of all surgical steps due to the EndoLIF platform technology
“With FDA clearance of two of our cages, our EndoLIF platform gets more complete; the next major step in endoscopic spinal surgery is endoscopically-assisted fusion,” said joimax Founder and CEO Wolfgang Ries. “More and more centers are proving the efficacy of these procedures in studies, demonstrating once again the major benefits of endoscopy in fusion. So far, our cages are well established in Europe and in selected centers in Asia, and now we are entering the USA, the biggest market for spinal fusion.”
Source: joimax
joimax received FDA 510(k) clearance to market the EndoLIF® Delta-Cage and EndoLIF® DoubleWedge-Cage. Delta-Cages and DoubleWedge-Cages are intended for intervertebral body fusion procedures for various diseases of the lumbar spine, such as degenerative disc disease.
Now in its 20th anniversary year, joimax sets another milestone by providing...
joimax received FDA 510(k) clearance to market the EndoLIF® Delta-Cage and EndoLIF® DoubleWedge-Cage. Delta-Cages and DoubleWedge-Cages are intended for intervertebral body fusion procedures for various diseases of the lumbar spine, such as degenerative disc disease.
Now in its 20th anniversary year, joimax sets another milestone by providing a family of 3D printed titanium cages. The complete EndoLIF product portfolio consists of the Delta-Cage, the DoubleWedge-Cage, and the O/On-Cage. All three models are designed with special features:
- 3D printed titanium for excellent bone ingrowth, fillable with bone or bone substitute material, pre- or post-insertion
- Anatomical shape and lordotic correction options for OLIF, TLIF, and PLIF procedures
- “Over-the-wire” implantation for optimal targeted positioning in the intervertebral disc space
- Endoscopic control of all surgical steps due to the EndoLIF platform technology
“With FDA clearance of two of our cages, our EndoLIF platform gets more complete; the next major step in endoscopic spinal surgery is endoscopically-assisted fusion,” said joimax Founder and CEO Wolfgang Ries. “More and more centers are proving the efficacy of these procedures in studies, demonstrating once again the major benefits of endoscopy in fusion. So far, our cages are well established in Europe and in selected centers in Asia, and now we are entering the USA, the biggest market for spinal fusion.”
Source: joimax

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







