Copy to clipboard
Copy to clipboard 
Intrinsic Therapeutics gained FDA Premarket Approval (PMA) for the Barricaid annular closure device for partial annulus replacement to treat herniated discs.
In late 2017, FDA’s Orthopaedic and Rehabilitation Devices Panel had recommended against approval of Barricaid, voting 9 to 5 that it is safe, 12 to 1 (with 1 abstaining) that it is effective in patients for whom it is indicated and 5 to 8 (with 1 abstaining) that its benefits outweigh the risks.
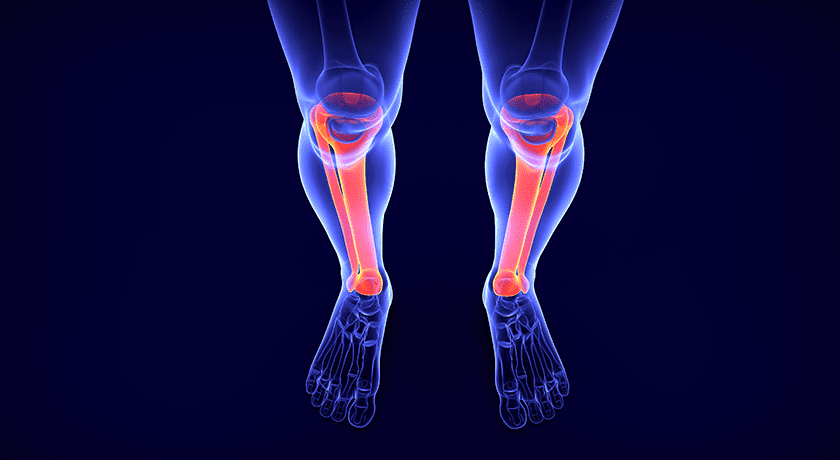
Barricaid is designed to close large defects in the annulus, enabling preservation of more of the patient’s disc. The device comprises a titanium bone anchor and self-expanding woven polymer mesh to seal the defect.
With PMA now in hand, Barricaid is indicated to reduce the incidence of reherniation and reoperation in skeletally mature patients with radiculopathy due to a posterior or posterolateral herniation following primary discectomy at a single level between L4 and S1.
Sources: MedTech Dive; FDA.gov; ORTHOWORLD, Inc.
Intrinsic Therapeutics gained FDA Premarket Approval (PMA) for the Barricaid annular closure device for partial annulus replacement to treat herniated discs.
In late 2017, FDA's Orthopaedic and Rehabilitation Devices Panel had recommended against approval of Barricaid, voting 9 to 5 that it is safe, 12 to 1 (with 1 abstaining) that it is...
Intrinsic Therapeutics gained FDA Premarket Approval (PMA) for the Barricaid annular closure device for partial annulus replacement to treat herniated discs.
In late 2017, FDA’s Orthopaedic and Rehabilitation Devices Panel had recommended against approval of Barricaid, voting 9 to 5 that it is safe, 12 to 1 (with 1 abstaining) that it is effective in patients for whom it is indicated and 5 to 8 (with 1 abstaining) that its benefits outweigh the risks.
Barricaid is designed to close large defects in the annulus, enabling preservation of more of the patient’s disc. The device comprises a titanium bone anchor and self-expanding woven polymer mesh to seal the defect.
With PMA now in hand, Barricaid is indicated to reduce the incidence of reherniation and reoperation in skeletally mature patients with radiculopathy due to a posterior or posterolateral herniation following primary discectomy at a single level between L4 and S1.
Sources: MedTech Dive; FDA.gov; ORTHOWORLD, Inc.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.