
 Copy to clipboard
Copy to clipboard 
Intrinsic Therapeutics announced that the American Medical Association CPT Editorial Panel accepted the addition of a new Category 1 CPT Code for bone-anchored annular closure. This is the first CPT Code to describe closing large annular defects using the Barricaid device.
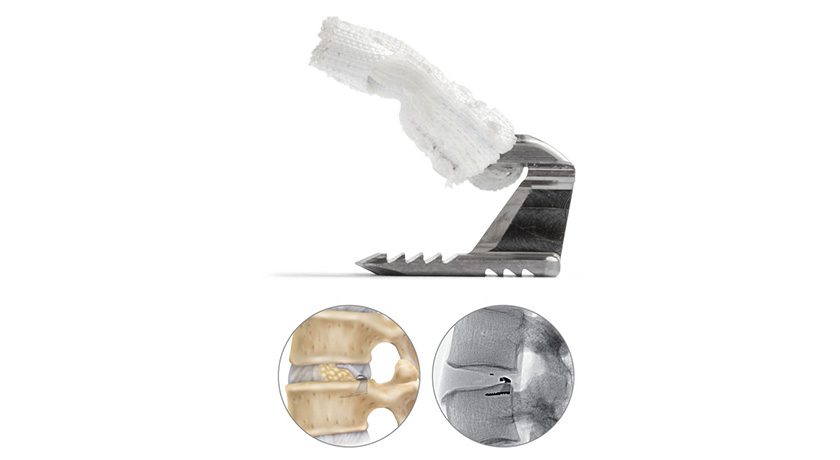
Barricaid is a proprietary technology designed to prevent reherniation and reoperation in patients with large annular defects following lumbar discectomy surgery. Barricaid has been implanted in more than eleven thousand patients and is supported by clinical studies in 8 distinct patient populations, including two randomized controlled trials and six single-armed trials.
This achievement of the Category 1 CPT code follows a succession of several key milestones for Barricaid including statistical superiority vs. discectomy alone in multiple randomized pivotal trials, FDA Premarket Approval, issuance by Center for Medicare & Medicaid Services of a clinical C-Code for facility payment, and Center for Disease Control publication of ICD-10-CM codes to report large annular defects. The addition of this CPT code represents a major reimbursement milestone for the Barricaid procedure that will help to standardize and streamline the reimbursement process. This code will go into effect on or after January 1, 2026.
“Successfully gaining the support and approval of key healthcare organizations like FDA, CMS, CDC and AMA requires irrefutable clinical evidence. We are truly grateful for the tremendous effort put forth by the surgeons and national specialty medical societies who have collaborated with one another and cooperated with Intrinsic to collect this clinical evidence and achieve these incredible milestones,” stated Ryan Drant, Chairman of the Board at Intrinsic Therapeutics.
Source: Intrinsic Therapeutics, Inc.
Intrinsic Therapeutics announced that the American Medical Association CPT Editorial Panel accepted the addition of a new Category 1 CPT Code for bone-anchored annular closure. This is the first CPT Code to describe closing large annular defects using the Barricaid device.
Barricaid is a proprietary technology designed to prevent reherniation...
Intrinsic Therapeutics announced that the American Medical Association CPT Editorial Panel accepted the addition of a new Category 1 CPT Code for bone-anchored annular closure. This is the first CPT Code to describe closing large annular defects using the Barricaid device.
Barricaid is a proprietary technology designed to prevent reherniation and reoperation in patients with large annular defects following lumbar discectomy surgery. Barricaid has been implanted in more than eleven thousand patients and is supported by clinical studies in 8 distinct patient populations, including two randomized controlled trials and six single-armed trials.
This achievement of the Category 1 CPT code follows a succession of several key milestones for Barricaid including statistical superiority vs. discectomy alone in multiple randomized pivotal trials, FDA Premarket Approval, issuance by Center for Medicare & Medicaid Services of a clinical C-Code for facility payment, and Center for Disease Control publication of ICD-10-CM codes to report large annular defects. The addition of this CPT code represents a major reimbursement milestone for the Barricaid procedure that will help to standardize and streamline the reimbursement process. This code will go into effect on or after January 1, 2026.
“Successfully gaining the support and approval of key healthcare organizations like FDA, CMS, CDC and AMA requires irrefutable clinical evidence. We are truly grateful for the tremendous effort put forth by the surgeons and national specialty medical societies who have collaborated with one another and cooperated with Intrinsic to collect this clinical evidence and achieve these incredible milestones,” stated Ryan Drant, Chairman of the Board at Intrinsic Therapeutics.
Source: Intrinsic Therapeutics, Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.