Copy to clipboard
Copy to clipboard 
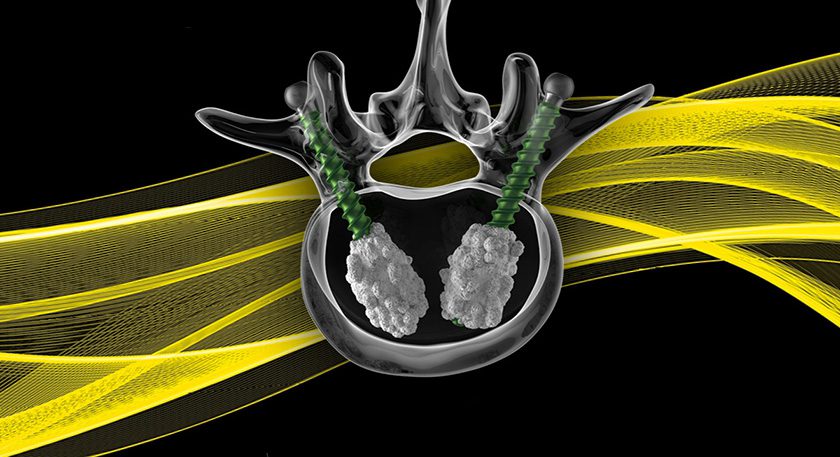
Intrinsic Therapeutics filed a Premarket Approval application with FDA for the Barricaid® Anular Closure device. The submission was based on 2-year outcomes of 554 trial subjects with a high risk of reherniation, subsequent reoperation and rehospitalization.
The Barricaid Anular Closure device is reported to be the first of its kind in a prospective randomized superiority trial involving patients who are at higher risk for revision discectomy to alleviate recurrent pain resulting from reherniation. Additional benefits include reduction in serious adverse events, episodic pain events and early hospital readmissions (at 30, 60 and 90 days).
The company completed enrollment for the trial and submitted the first of four PMA application modules at the end of 2014.
Sources: Intrinsic Therapeutics, Inc.; ORTHOWORLD Inc.
Intrinsic Therapeutics filed a Premarket Approval application with FDA for the Barricaid® Anular Closure device. The submission was based on 2-year outcomes of 554 trial subjects with a high risk of reherniation, subsequent reoperation and rehospitalization.
The Barricaid Anular Closure device is reported to be the first of its kind in a...
Intrinsic Therapeutics filed a Premarket Approval application with FDA for the Barricaid® Anular Closure device. The submission was based on 2-year outcomes of 554 trial subjects with a high risk of reherniation, subsequent reoperation and rehospitalization.
The Barricaid Anular Closure device is reported to be the first of its kind in a prospective randomized superiority trial involving patients who are at higher risk for revision discectomy to alleviate recurrent pain resulting from reherniation. Additional benefits include reduction in serious adverse events, episodic pain events and early hospital readmissions (at 30, 60 and 90 days).
The company completed enrollment for the trial and submitted the first of four PMA application modules at the end of 2014.
Sources: Intrinsic Therapeutics, Inc.; ORTHOWORLD Inc.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.