
 Copy to clipboard
Copy to clipboard 
Integrity Orthopaedics closed on a Series B financing of $20.6 million. The capital will expand U.S. commercial launch of its initial product in the rotator cuff repair market.
The Integrity Orthopaedics system is designed to address the biggest unmet need in rotator cuff repairs, reducing the occurrence of cuff re-tears following surgical repair. Current surgical techniques have a structural failure rate that typically averages 20-40%, with rates ranging from 8-94% in specific studies (Young, et al., 2023).
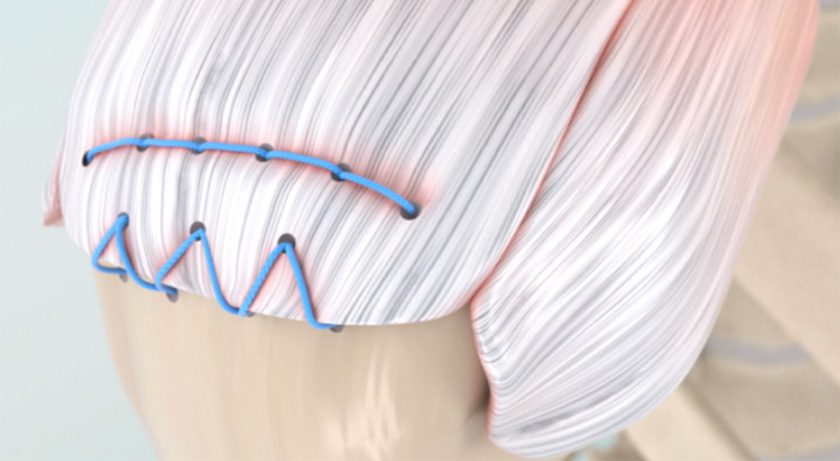
The Integrity Orthopaedics system was FDA-cleared in early 2023 and initially utilized by a small group of surgeons in a limited launch. This system uses patented micro-anchors, a continuous, locking stitch, a patented instrument for anchor installation and operative techniques to enable physicians to improve the fixation and healing of torn tendons.
Integrity Orthopaedics was co-founded in 2020 by Thomas Westling, a veteran executive in medtech and previously Founder and CEO of Rotation Medical, David Crompton, Chief IP and General Counsel and orthopedic surgeons Dr. Patrick Connor, Dr. Howard Harris and Dr. Marc Labbe.
“We are grateful for the support of our shareholders, who we view as a strategic asset,” said Thomas Westling. “We have been blessed with an extraordinary set of investors, many with deep health care expertise and connections throughout the health care ecosystem, and they have consistently given us valuable counsel since our founding.”
Source: Integrity Orthopaedics
Integrity Orthopaedics closed on a Series B financing of $20.6 million. The capital will expand U.S. commercial launch of its initial product in the rotator cuff repair market.
The Integrity Orthopaedics system is designed to address the biggest unmet need in rotator cuff repairs, reducing the occurrence of cuff re-tears following surgical...
Integrity Orthopaedics closed on a Series B financing of $20.6 million. The capital will expand U.S. commercial launch of its initial product in the rotator cuff repair market.
The Integrity Orthopaedics system is designed to address the biggest unmet need in rotator cuff repairs, reducing the occurrence of cuff re-tears following surgical repair. Current surgical techniques have a structural failure rate that typically averages 20-40%, with rates ranging from 8-94% in specific studies (Young, et al., 2023).
The Integrity Orthopaedics system was FDA-cleared in early 2023 and initially utilized by a small group of surgeons in a limited launch. This system uses patented micro-anchors, a continuous, locking stitch, a patented instrument for anchor installation and operative techniques to enable physicians to improve the fixation and healing of torn tendons.
Integrity Orthopaedics was co-founded in 2020 by Thomas Westling, a veteran executive in medtech and previously Founder and CEO of Rotation Medical, David Crompton, Chief IP and General Counsel and orthopedic surgeons Dr. Patrick Connor, Dr. Howard Harris and Dr. Marc Labbe.
“We are grateful for the support of our shareholders, who we view as a strategic asset,” said Thomas Westling. “We have been blessed with an extraordinary set of investors, many with deep health care expertise and connections throughout the health care ecosystem, and they have consistently given us valuable counsel since our founding.”
Source: Integrity Orthopaedics

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







