
 Copy to clipboard
Copy to clipboard 
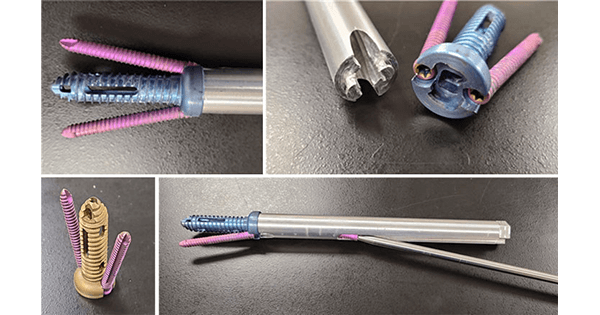
Inspired Spine was granted FDA 510(k) clearance to market Trident Sacroiliac (SI) Joint Screw, designed to improve procedure efficiency resulting in surgery durations less than 15 minutes.
The system employs one main screw and two integrated side screws to deliver three distinct screw approaches into a single trajectory. Trident’s integrated delivery and implant system comprises a multi-use kit with a limited number of instruments, replacing multiple component instrument trays currently employed by other SI Fusion systems.
Its main screw delivery sleeve provides a targeting guide for side screw delivery, improving procedure efficiency and optimizing imaging requirements. The single incision and corresponding preplanned fixed trajectory decreases required procedure time and radiation exposure. The Trident SI screw design includes self-tapping graft collection flutes which reduce the need for additional biologic while delivering bone graft.
Inspired Spine was granted FDA 510(k) clearance to market Trident Sacroiliac (SI) Joint Screw, designed to improve procedure efficiency resulting in surgery durations less than 15 minutes.
The system employs one main screw and two integrated side screws to deliver three distinct screw approaches into a single trajectory. Trident's...
Inspired Spine was granted FDA 510(k) clearance to market Trident Sacroiliac (SI) Joint Screw, designed to improve procedure efficiency resulting in surgery durations less than 15 minutes.
The system employs one main screw and two integrated side screws to deliver three distinct screw approaches into a single trajectory. Trident’s integrated delivery and implant system comprises a multi-use kit with a limited number of instruments, replacing multiple component instrument trays currently employed by other SI Fusion systems.
Its main screw delivery sleeve provides a targeting guide for side screw delivery, improving procedure efficiency and optimizing imaging requirements. The single incision and corresponding preplanned fixed trajectory decreases required procedure time and radiation exposure. The Trident SI screw design includes self-tapping graft collection flutes which reduce the need for additional biologic while delivering bone graft.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







