
 Copy to clipboard
Copy to clipboard 
 INSPAN announced successful results from a long-term follow-up clinical study of outpatient L4-L5 lumbar interspinous fixation for degenerative spinal stenosis using the Inspan® ScrewLES™ Fusion interspinous fixation device.
INSPAN announced successful results from a long-term follow-up clinical study of outpatient L4-L5 lumbar interspinous fixation for degenerative spinal stenosis using the Inspan® ScrewLES™ Fusion interspinous fixation device.
Unlike extension block designs, Inspan fixates the spine to allow for immediate stability, distraction, decompression and fusion, and is an alternative to the traditional method that uses pedicle screws and interbodies with laminectomies.
The retrospective review of prospectively collected data evaluated 122 surgical cases of lumbar decompression with interspinous fixation. Fifty-six patients had instrumentation at L4-L5. Two-year VAS and ODI showed significant improvement from 8.1+/-1.2 to 1.5+/-1.1 and 42.9+/-14.3 to 14.8+/-5.1. All surgeries were completed in under one hour. There was one revision case with removal of Inspan and converted to an open hemilaminectomy decompression.
The long-term results of the study demonstrated improved outcomes in patients who underwent interspinous distraction decompression in an ambulatory surgery center using the Inspan device at L4-L5 for degenerative spinal stenosis. There were no complications or implant failures.
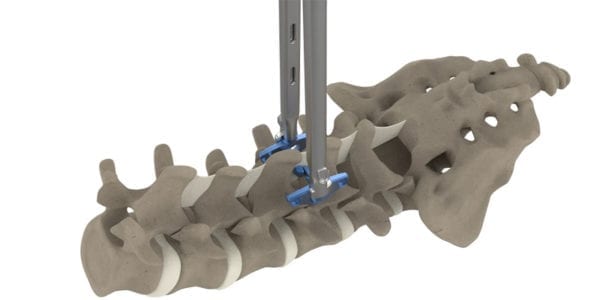
The Inspan Slim Spinous Process Plate is a posterior non-pedicle supplemental fixation system intended for use in the non-cervical spine (T1-S1). It is intended for plate fixation/attachment to the spinous process to achieve supplemental fusion for spondylolisthesis, trauma, tumor or degenerative disc disease. The device is intended for use with bone graft material and is not intended for standalone use.
INSPAN announced successful results from a long-term follow-up clinical study of outpatient L4-L5 lumbar interspinous fixation for degenerative spinal stenosis using the Inspan® ScrewLES™ Fusion interspinous fixation device.
Unlike extension block designs, Inspan fixates the spine to allow for immediate stability, distraction,...
 INSPAN announced successful results from a long-term follow-up clinical study of outpatient L4-L5 lumbar interspinous fixation for degenerative spinal stenosis using the Inspan® ScrewLES™ Fusion interspinous fixation device.
INSPAN announced successful results from a long-term follow-up clinical study of outpatient L4-L5 lumbar interspinous fixation for degenerative spinal stenosis using the Inspan® ScrewLES™ Fusion interspinous fixation device.
Unlike extension block designs, Inspan fixates the spine to allow for immediate stability, distraction, decompression and fusion, and is an alternative to the traditional method that uses pedicle screws and interbodies with laminectomies.
The retrospective review of prospectively collected data evaluated 122 surgical cases of lumbar decompression with interspinous fixation. Fifty-six patients had instrumentation at L4-L5. Two-year VAS and ODI showed significant improvement from 8.1+/-1.2 to 1.5+/-1.1 and 42.9+/-14.3 to 14.8+/-5.1. All surgeries were completed in under one hour. There was one revision case with removal of Inspan and converted to an open hemilaminectomy decompression.
The long-term results of the study demonstrated improved outcomes in patients who underwent interspinous distraction decompression in an ambulatory surgery center using the Inspan device at L4-L5 for degenerative spinal stenosis. There were no complications or implant failures.
The Inspan Slim Spinous Process Plate is a posterior non-pedicle supplemental fixation system intended for use in the non-cervical spine (T1-S1). It is intended for plate fixation/attachment to the spinous process to achieve supplemental fusion for spondylolisthesis, trauma, tumor or degenerative disc disease. The device is intended for use with bone graft material and is not intended for standalone use.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







