
 Copy to clipboard
Copy to clipboard 
Insight Surgery, a personalized surgery technology company, completed a $2.5 million raise led by Nodenza Venture Partners.
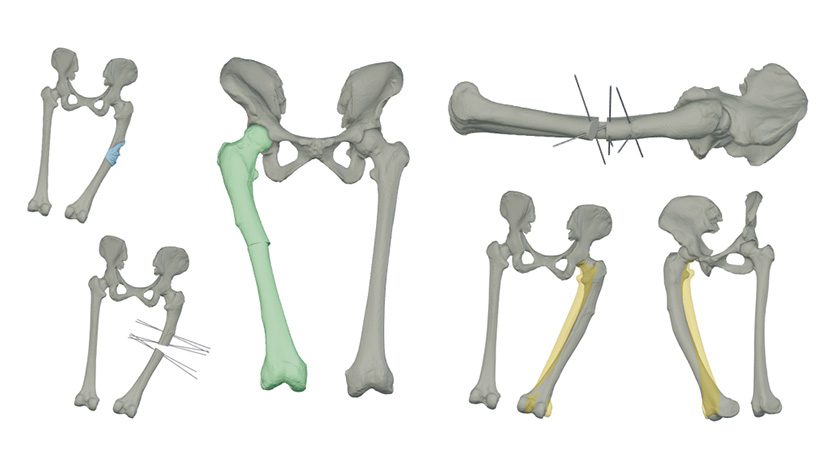
This investment follows the granting of FDA 510(k) clearance for the company’s surgical guides for orthopedic and orthopedic-oncology surgery, and will be used to accelerate growth in the U.S. market. Their planned expansion will provide surgeons with rapid access to tools that improve patient outcomes, reduce risk and expedite recovery.
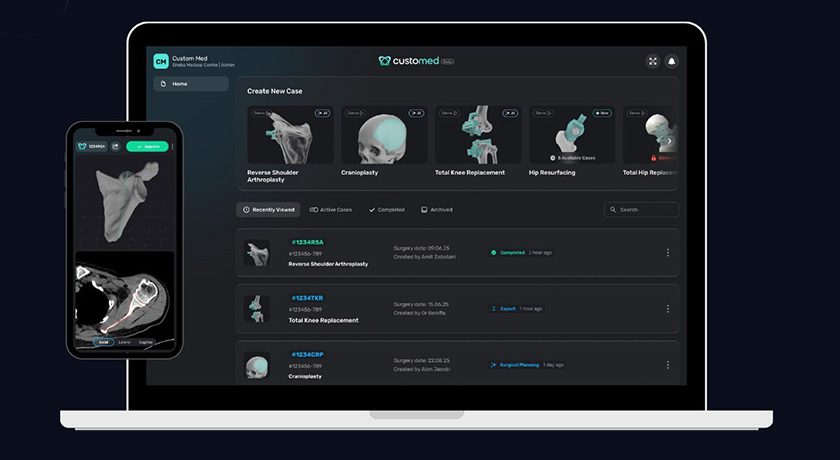
Insight’s proprietary digital platform, EmbedMed, digitizes the surgical planning process and allows the rapid design and manufacture of the patient-specific guides for orthopedic surgery.
Functions include the processing of patient scan data and as a pre-operative software tool for simulating and evaluating surgical planning options. The output files from the system can be provided digitally, or as physical models and surgical guides. The nature of the platform enables Surgical Guides to be provided to surgeons in 10 working days from Insight’s ISO 13485-certified and FDA-registered facilities in the U.S.
Insight Surgery recently launched their U.S. business with the opening of a cleanroom manufacturing facility at the Texas Medical Centre (TMC), Houston. They have since gained considerable market traction, entering into a strategic partnership with Ricoh USA and partnering with surgeons at several major hospitals across the U.S., including the Hospital for Special Surgery in New York, University Hospitals of Chicago, University of Florida Health, and UAB in Birmingham Alabama.
Their Series A raise, and the support of the Nodenza team, will unlock expansion opportunities for Insight Surgery across the U.S., including the opening of two new manufacturing facilities at East and West-coast hospital sites. The company has plans to double headcount in the U.S. in the coming months.
Henry Pinchbeck, Chief Executive Officer at Insight Surgery, said, “Our mission is to make advanced surgical planning tools accessible and scalable across the U.S. healthcare system. This investment allows us to accelerate our plan to enable every orthopedic surgeon in the U.S. to have easy access to Personalized Surgical devices within surgically meaningful timelines. We’re excited to build on the momentum we’ve already seen with leading clinicians. We’re not just focused on improving surgical precision – we’re focused on doing it in a way that makes financial sense for healthcare systems. By reducing operating time, streamlining workflows, and lowering complication and readmission rates, our technology can help hospitals drive down costs while delivering better care. This investment allows us to scale that impact nationwide.”
Source: Insight Surgery
Insight Surgery, a personalized surgery technology company, completed a $2.5 million raise led by Nodenza Venture Partners.
This investment follows the granting of FDA 510(k) clearance for the company’s surgical guides for orthopedic and orthopedic-oncology surgery, and will be used to accelerate growth in the U.S. market. Their planned...
Insight Surgery, a personalized surgery technology company, completed a $2.5 million raise led by Nodenza Venture Partners.
This investment follows the granting of FDA 510(k) clearance for the company’s surgical guides for orthopedic and orthopedic-oncology surgery, and will be used to accelerate growth in the U.S. market. Their planned expansion will provide surgeons with rapid access to tools that improve patient outcomes, reduce risk and expedite recovery.
Insight’s proprietary digital platform, EmbedMed, digitizes the surgical planning process and allows the rapid design and manufacture of the patient-specific guides for orthopedic surgery.
Functions include the processing of patient scan data and as a pre-operative software tool for simulating and evaluating surgical planning options. The output files from the system can be provided digitally, or as physical models and surgical guides. The nature of the platform enables Surgical Guides to be provided to surgeons in 10 working days from Insight’s ISO 13485-certified and FDA-registered facilities in the U.S.
Insight Surgery recently launched their U.S. business with the opening of a cleanroom manufacturing facility at the Texas Medical Centre (TMC), Houston. They have since gained considerable market traction, entering into a strategic partnership with Ricoh USA and partnering with surgeons at several major hospitals across the U.S., including the Hospital for Special Surgery in New York, University Hospitals of Chicago, University of Florida Health, and UAB in Birmingham Alabama.
Their Series A raise, and the support of the Nodenza team, will unlock expansion opportunities for Insight Surgery across the U.S., including the opening of two new manufacturing facilities at East and West-coast hospital sites. The company has plans to double headcount in the U.S. in the coming months.
Henry Pinchbeck, Chief Executive Officer at Insight Surgery, said, “Our mission is to make advanced surgical planning tools accessible and scalable across the U.S. healthcare system. This investment allows us to accelerate our plan to enable every orthopedic surgeon in the U.S. to have easy access to Personalized Surgical devices within surgically meaningful timelines. We’re excited to build on the momentum we’ve already seen with leading clinicians. We’re not just focused on improving surgical precision – we’re focused on doing it in a way that makes financial sense for healthcare systems. By reducing operating time, streamlining workflows, and lowering complication and readmission rates, our technology can help hospitals drive down costs while delivering better care. This investment allows us to scale that impact nationwide.”
Source: Insight Surgery

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.