
 Copy to clipboard
Copy to clipboard 
Inovedis received FDA clearance to market the SINEFIX implant system for the repair of rotator cuff tears.
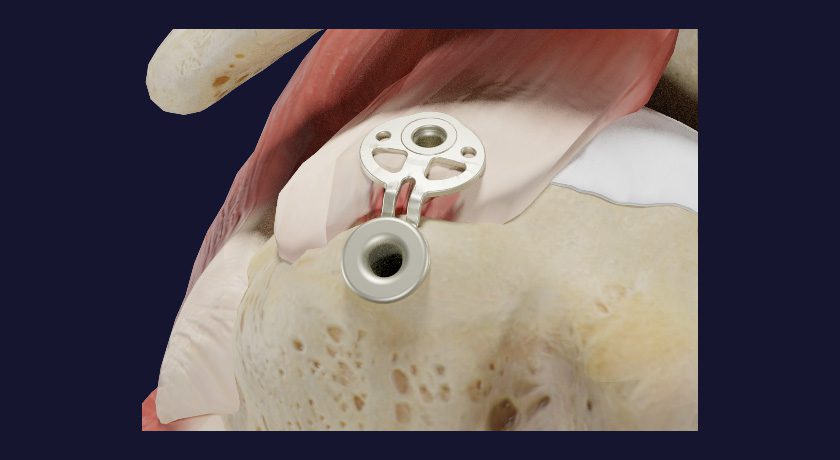
The SINEFIX implant system allows refixation of the rotator cuff tendon to bone with a simplified surgical technique that has been optimized for minimally invasive surgery. The technique aims to significantly reduce time and cost of the procedure.
Unlike the traditional surgery, the new technique provides effective mechanical reinforcement of the fixed tendon and stimulates the patient’s intrinsic healing potential. SINEFIX creates a flat and even contact of tendon and bone. It distributes the shear stress uniformly and does not cause punctual pressure peaks, while also maintaining blood circulation and supporting the healing process.
The insertion of the implant is possible with a two-step technique: The base plate is positioned, and the fixation points are driven into the bone with a hammer and the appropriate SINEFIX instruments provided by Inovedis. No preliminary work is needed, and the device may be implanted using arthroscopic techniques or open reconstruction. This simplified technique is designed to reduce the risk of complications due to surgical errors and significantly shortens surgery time.
“With FDA clearance we will now initiate a first mover application to generate real-world experience in the U.S. to evaluate the potential of the technology,” said Lukas Flöss, Founder and CEO.
Product launch will occur this month at the American Orthopaedic Society for Sports Medicine (AOSSM) annual meeting, followed by enrollment of clinical sites into a limited market release.
Source: Inovedis GmbH
Inovedis received FDA clearance to market the SINEFIX implant system for the repair of rotator cuff tears.
The SINEFIX implant system allows refixation of the rotator cuff tendon to bone with a simplified surgical technique that has been optimized for minimally invasive surgery. The technique aims to significantly reduce time and cost of the...
Inovedis received FDA clearance to market the SINEFIX implant system for the repair of rotator cuff tears.
The SINEFIX implant system allows refixation of the rotator cuff tendon to bone with a simplified surgical technique that has been optimized for minimally invasive surgery. The technique aims to significantly reduce time and cost of the procedure.
Unlike the traditional surgery, the new technique provides effective mechanical reinforcement of the fixed tendon and stimulates the patient’s intrinsic healing potential. SINEFIX creates a flat and even contact of tendon and bone. It distributes the shear stress uniformly and does not cause punctual pressure peaks, while also maintaining blood circulation and supporting the healing process.
The insertion of the implant is possible with a two-step technique: The base plate is positioned, and the fixation points are driven into the bone with a hammer and the appropriate SINEFIX instruments provided by Inovedis. No preliminary work is needed, and the device may be implanted using arthroscopic techniques or open reconstruction. This simplified technique is designed to reduce the risk of complications due to surgical errors and significantly shortens surgery time.
“With FDA clearance we will now initiate a first mover application to generate real-world experience in the U.S. to evaluate the potential of the technology,” said Lukas Flöss, Founder and CEO.
Product launch will occur this month at the American Orthopaedic Society for Sports Medicine (AOSSM) annual meeting, followed by enrollment of clinical sites into a limited market release.
Source: Inovedis GmbH

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







