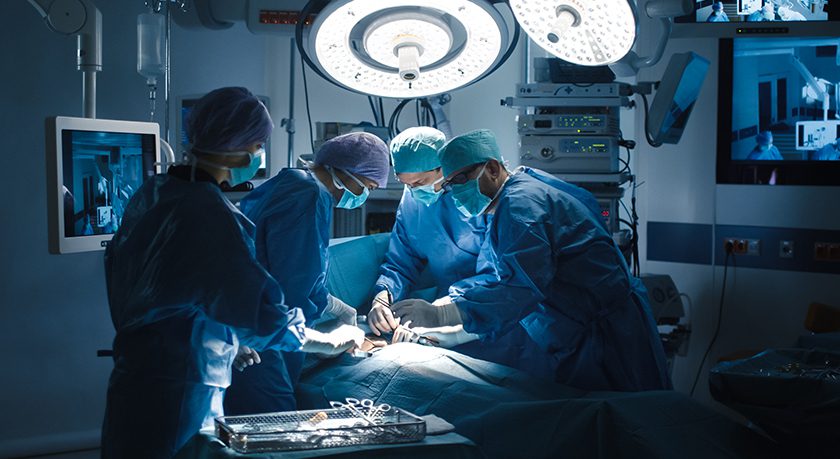
Though wireless technology has swept the globe in consumer devices, its use in the operating room has lagged. Surgeons use devices and equipment that are wired to each other and to power and light sources. The prevalence of cords in the O.R. introduces the opportunity for contamination, accidents and even fire.
Recognizing these issues, Indago has worked closely with orthopedic surgeons to design a patented, integrated, wireless arthroscopic camera system.
“We believe ArthroFree will be the world’s first fully wireless, minimally invasive surgical camera,” said Daniel Dudley, co-founder and Chief Operating Officer of Indago. “It is a wireless arthroscopic camera for hip, knee and shoulder surgeries. We have developed a number of fundamental technologies that allow us to eliminate the cables that are currently endemic to endoscopic procedures.”
The Difference in the Details
Typical arthroscopes have a handheld camera with a video power cable that connects to a rigid scope. The scope is then connected via fiber optic cable to an external light source. Some companies are developing equipment that reduces the number of wires in a system, but have not entirely eliminated them, Dudley said, or they are being used for diagnostics instead of surgical procedures. Other companies have created wireless systems, but the image quality is not at the level needed for surgery.
Major arthroscopic equipment challenges in the O.R. include the number of wires that get in the way of the surgical team, the potential for infection and additional sterilization needs that cables pose, and the risk of fire or other accidents.
ArthroFree utilizes a patented, novel light source that addresses the problem of fires in the O.R. caused by overheating. One of the big challenges with current arthroscopic camera systems is the inability to leave the light on outside of the body because the light produces an immense amount of heat.
“You’re in an oxygen-rich environment, there are paper drapes, and increasingly we have alcohol-based disinfection, so there is a real potential for a flash fire scenario,” Dudley said. “Scopes get very hot because of the amount of light they transmit, so we wanted to avoid that. There have been advances in technology, but there still is a direct correlation between the amount of light output and the amount of heat output.”
Looking to solve the issue, the Indago team worked with their Chief Optical Scientist Howard Fein, who has decades of experience in developing lighting and photonics systems. Together, they created a miniaturized, integrated light source that produces a comparable amount of light to an external light source with far less power and nominal heat.
Furthermore, safety from a sterilization perspective has been a key focus for Indago when developing ArthroFree. FDA previously released guidance on the unfortunate deaths due to duodenoscope infections, with particular mention of the difficulty of sterilizing endoscopic cables.
“That was something that we had been watching very carefully,” Dudley said. “We wanted to make a more efficient camera system to help improve the quality of care, but also a camera that could be safer because that’s critically important to us. We’ve taken that guidance to heart.”
Industry discussion has also centered on the use of old predicates and movement to predicates that are less than 10 years old as the basis for an FDA 510(k) exempt device. With safety at the forefront, Indago kept that in mind as they developed ArthroFree.
“I would say that we are taking the most aggressive stance from an FDA perspective,” Dudley said. “If FDA suggests you do it, or is looking like they’re moving in a direction, we are already there, because when it comes to safety and regulatory compliance, we want to be future-proof.”
From the beginning, Indago also focused on making sure that surgeons were involved in the product development process.
“Too many times, as an industry, we’ve seen mistakes made when we are overly reliant on focus groups or marketers,” Dudley said. “We’ve been designing this from the beginning with the end user not only in mind, but integral to the development process.”
This collaborative approach has produced an ergonomic product with comprehensive scope compatibility meant to integrate into the O.R. seamlessly. The device is drop-in compatible with existing systems to reduce friction for implementation. And it’s an economical option that doesn’t require replacing all of the equipment already in use in the O.R.
“One of the things that’s critical to us is that we’re bringing clinically relevant devices with new or adapted technologies into the operating room, and we’re doing it in such a way that we believe will mitigate the financial impact on hospitals and private practices,” Dudley said. “We view this device as the first step in opening the door to the operating room of the future. We don’t want to lock the technology in a walled garden and charge people through the nose for it. We take a rising-tide-lifts-all-boats approach.”
Market Opportunities
Indago expects to seek FDA clearance for ArthroFree in 4Q of 2020, with market entry in 2021. The first marketing opportunity will be focused on private practice physicians who want to increase their O.R. utilization, decrease procedure time and reduce cost.
“In an outpatient or an ambulatory surgical center, we expect that the customer will be a physician owner that likely only gets paid when they do procedures,” Dudley said. “We have an opportunity to come in and show them that ArthroFree may drive efficiency gains and differentiate their practice in a crowded field.
“We believe that once institutions begin using ArthroFree in their operating rooms, it will be a no-brainer for them,” Dudley said. “We expect that it will reduce their supply chain and their purchasing management. They will also have far fewer items to transport between operating rooms. ArthroFree’s central processing and sterilization requirements are significantly less than competitor devices because it doesn’t have all of these cables, which are known vectors for potential infection.”
Broadly speaking about the ArthroFree technology, it is an arthroscopic camera. But fundamentally, it is a minimally invasive camera platform. Therefore, while ArthroFree is targeted explicitly at arthroscopic sports medicine, Indago is already discussing other endoscopic surgeries — initially rigid scope like OB-GYN, ENT and small joint, and then potentially moving into flexible scope endoscopies.
“We are already developing the next generation of ArthroFree with 4K imaging and looking at other technologies that are beginning to be adopted across the operating room,” Dudley
said. “We’re definitely not standing still. ArthroFree is our first product, but we see it as the first in a family of products that can help move these procedures toward what we view as the future.”
In the near term, Dudley expects that Indago will expand upon and double down on their strategy of bringing new technologies from different industries like automotive and defense into healthcare applications.
“We want to bring technologies into healthcare from industries that have similar concerns, that are highly regulated, secure and need to be robust as a way to move the industry forward,” Dudley said. “We’ve got a number of projects in the pipeline that we want to bring to market, and that will be a huge push for us over the next several years.”
Though wireless technology has swept the globe in consumer devices, its use in the operating room has lagged. Surgeons use devices and equipment that are wired to each other and to power and light sources. The prevalence of cords in the O.R. introduces the opportunity for contamination, accidents and even fire.
Recognizing these issues, Indago...
Though wireless technology has swept the globe in consumer devices, its use in the operating room has lagged. Surgeons use devices and equipment that are wired to each other and to power and light sources. The prevalence of cords in the O.R. introduces the opportunity for contamination, accidents and even fire.
Recognizing these issues, Indago has worked closely with orthopedic surgeons to design a patented, integrated, wireless arthroscopic camera system.
“We believe ArthroFree will be the world’s first fully wireless, minimally invasive surgical camera,” said Daniel Dudley, co-founder and Chief Operating Officer of Indago. “It is a wireless arthroscopic camera for hip, knee and shoulder surgeries. We have developed a number of fundamental technologies that allow us to eliminate the cables that are currently endemic to endoscopic procedures.”
The Difference in the Details
Typical arthroscopes have a handheld camera with a video power cable that connects to a rigid scope. The scope is then connected via fiber optic cable to an external light source. Some companies are developing equipment that reduces the number of wires in a system, but have not entirely eliminated them, Dudley said, or they are being used for diagnostics instead of surgical procedures. Other companies have created wireless systems, but the image quality is not at the level needed for surgery.
Major arthroscopic equipment challenges in the O.R. include the number of wires that get in the way of the surgical team, the potential for infection and additional sterilization needs that cables pose, and the risk of fire or other accidents.
ArthroFree utilizes a patented, novel light source that addresses the problem of fires in the O.R. caused by overheating. One of the big challenges with current arthroscopic camera systems is the inability to leave the light on outside of the body because the light produces an immense amount of heat.
“You’re in an oxygen-rich environment, there are paper drapes, and increasingly we have alcohol-based disinfection, so there is a real potential for a flash fire scenario,” Dudley said. “Scopes get very hot because of the amount of light they transmit, so we wanted to avoid that. There have been advances in technology, but there still is a direct correlation between the amount of light output and the amount of heat output.”
Looking to solve the issue, the Indago team worked with their Chief Optical Scientist Howard Fein, who has decades of experience in developing lighting and photonics systems. Together, they created a miniaturized, integrated light source that produces a comparable amount of light to an external light source with far less power and nominal heat.
Furthermore, safety from a sterilization perspective has been a key focus for Indago when developing ArthroFree. FDA previously released guidance on the unfortunate deaths due to duodenoscope infections, with particular mention of the difficulty of sterilizing endoscopic cables.
“That was something that we had been watching very carefully,” Dudley said. “We wanted to make a more efficient camera system to help improve the quality of care, but also a camera that could be safer because that’s critically important to us. We’ve taken that guidance to heart.”
Industry discussion has also centered on the use of old predicates and movement to predicates that are less than 10 years old as the basis for an FDA 510(k) exempt device. With safety at the forefront, Indago kept that in mind as they developed ArthroFree.
“I would say that we are taking the most aggressive stance from an FDA perspective,” Dudley said. “If FDA suggests you do it, or is looking like they’re moving in a direction, we are already there, because when it comes to safety and regulatory compliance, we want to be future-proof.”
From the beginning, Indago also focused on making sure that surgeons were involved in the product development process.
“Too many times, as an industry, we’ve seen mistakes made when we are overly reliant on focus groups or marketers,” Dudley said. “We’ve been designing this from the beginning with the end user not only in mind, but integral to the development process.”
This collaborative approach has produced an ergonomic product with comprehensive scope compatibility meant to integrate into the O.R. seamlessly. The device is drop-in compatible with existing systems to reduce friction for implementation. And it’s an economical option that doesn’t require replacing all of the equipment already in use in the O.R.
“One of the things that’s critical to us is that we’re bringing clinically relevant devices with new or adapted technologies into the operating room, and we’re doing it in such a way that we believe will mitigate the financial impact on hospitals and private practices,” Dudley said. “We view this device as the first step in opening the door to the operating room of the future. We don’t want to lock the technology in a walled garden and charge people through the nose for it. We take a rising-tide-lifts-all-boats approach.”
Market Opportunities
Indago expects to seek FDA clearance for ArthroFree in 4Q of 2020, with market entry in 2021. The first marketing opportunity will be focused on private practice physicians who want to increase their O.R. utilization, decrease procedure time and reduce cost.
“In an outpatient or an ambulatory surgical center, we expect that the customer will be a physician owner that likely only gets paid when they do procedures,” Dudley said. “We have an opportunity to come in and show them that ArthroFree may drive efficiency gains and differentiate their practice in a crowded field.
“We believe that once institutions begin using ArthroFree in their operating rooms, it will be a no-brainer for them,” Dudley said. “We expect that it will reduce their supply chain and their purchasing management. They will also have far fewer items to transport between operating rooms. ArthroFree’s central processing and sterilization requirements are significantly less than competitor devices because it doesn’t have all of these cables, which are known vectors for potential infection.”
Broadly speaking about the ArthroFree technology, it is an arthroscopic camera. But fundamentally, it is a minimally invasive camera platform. Therefore, while ArthroFree is targeted explicitly at arthroscopic sports medicine, Indago is already discussing other endoscopic surgeries — initially rigid scope like OB-GYN, ENT and small joint, and then potentially moving into flexible scope endoscopies.
“We are already developing the next generation of ArthroFree with 4K imaging and looking at other technologies that are beginning to be adopted across the operating room,” Dudley
said. “We’re definitely not standing still. ArthroFree is our first product, but we see it as the first in a family of products that can help move these procedures toward what we view as the future.”
In the near term, Dudley expects that Indago will expand upon and double down on their strategy of bringing new technologies from different industries like automotive and defense into healthcare applications.
“We want to bring technologies into healthcare from industries that have similar concerns, that are highly regulated, secure and need to be robust as a way to move the industry forward,” Dudley said. “We’ve got a number of projects in the pipeline that we want to bring to market, and that will be a huge push for us over the next several years.”


You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
HT
Heather Tunstall is an ORTHOWORLD Contributor and owner of Tunstall Content.