
 Copy to clipboard
Copy to clipboard 
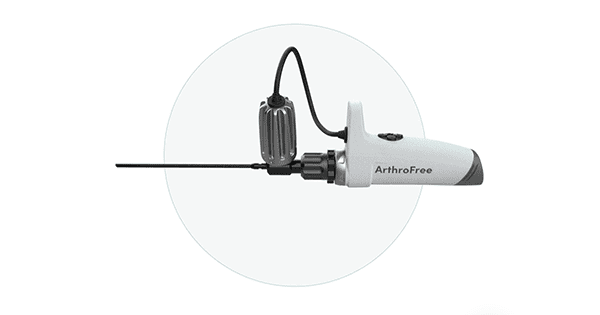
Indago closed its $10 million convertible note round of financing. The funds will be used to complete pre-submission testing of the ArthroFree™ – the company’s transformative wireless camera system – and to submit the device for FDA clearance, as well as continuing to develop a diverse pipeline of additional products and expand the company’s intellectual property portfolio. The company plans to submit its 510(k) premarket notification in late 2021 and expects to receive FDA approval to launch the product in the first half of 2022.
The company also announced that it is changing its name to Lazurite Holdings, effective immediately.
“We are excited to have closed the financing round and look forward to using the funds to help move ArthroFree through the FDA and continuing the development of our suite of follow-on products,” said Eugene Malinskiy, Chief Executive Officer and founder. “With the close of the financing round, we also are excited to announce the company’s new name and to welcome Michael Salerno to our Board. Michael’s depth of knowledge and experience in capital raising in the health care company space will be invaluable to us as we continue to grow Lazurite and expand our product base.”
He continued, “Our novel and patented Meridiem™ light engine technology is one of our core assets. It is the basis for our ArthroFree wireless arthroscopic camera system as well as a number of other products in our pipeline. When people see or hear the name Lazurite™ — which references the color of the light emitted from the laser diode in our low-heat, high power light engine technology – we would like them to think of us as the medical device and innovations company known for its transformative surgical wireless platform, laser-based lighting and other advanced technologies.”
Mark Froimson, MD, Chair of the Board of Managers of Lazurite, said, “We are very pleased to have completed another successful capital raise and to welcome Michael Salerno to our Board. His experience leading a venture cap fund focused on health care-oriented companies will be particularly valuable. We are looking forward to showcasing our products and the upcoming FDA submission.”
Source: Lazurite
Indago closed its $10 million convertible note round of financing. The funds will be used to complete pre-submission testing of the ArthroFree™ – the company’s transformative wireless camera system – and to submit the device for FDA clearance, as well as continuing to develop a diverse pipeline of additional products and expand the company’s...
Indago closed its $10 million convertible note round of financing. The funds will be used to complete pre-submission testing of the ArthroFree™ – the company’s transformative wireless camera system – and to submit the device for FDA clearance, as well as continuing to develop a diverse pipeline of additional products and expand the company’s intellectual property portfolio. The company plans to submit its 510(k) premarket notification in late 2021 and expects to receive FDA approval to launch the product in the first half of 2022.
The company also announced that it is changing its name to Lazurite Holdings, effective immediately.
“We are excited to have closed the financing round and look forward to using the funds to help move ArthroFree through the FDA and continuing the development of our suite of follow-on products,” said Eugene Malinskiy, Chief Executive Officer and founder. “With the close of the financing round, we also are excited to announce the company’s new name and to welcome Michael Salerno to our Board. Michael’s depth of knowledge and experience in capital raising in the health care company space will be invaluable to us as we continue to grow Lazurite and expand our product base.”
He continued, “Our novel and patented Meridiem™ light engine technology is one of our core assets. It is the basis for our ArthroFree wireless arthroscopic camera system as well as a number of other products in our pipeline. When people see or hear the name Lazurite™ — which references the color of the light emitted from the laser diode in our low-heat, high power light engine technology – we would like them to think of us as the medical device and innovations company known for its transformative surgical wireless platform, laser-based lighting and other advanced technologies.”
Mark Froimson, MD, Chair of the Board of Managers of Lazurite, said, “We are very pleased to have completed another successful capital raise and to welcome Michael Salerno to our Board. His experience leading a venture cap fund focused on health care-oriented companies will be particularly valuable. We are looking forward to showcasing our products and the upcoming FDA submission.”
Source: Lazurite

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







